Gene silencing technique offers new strategy for treating, curing disease
A new technique aimed at directly controlling the Expression of genes by turning them on or off at the DNA level could lead to drugs for the treatment or cure of many diseases, say researchers at UT Southwestern Medical Center.
"Virtually every disease starts at the level of malfunctioning gene expression, or viral or bacterial gene expression," said Dr. David Corey, professor of pharmacology and biochemistry. "This is an approach that could theoretically produce a drug for the treatment or cure of almost any disease."
In two papers appearing in the online edition of the journal Nature Chemical Biology, Dr. Corey and his colleagues describe how they efficiently shut down gene expression in cultured cells by blocking the ability of chromosomal DNA to be copied into RNA and made into proteins. The studies, which Dr. Corey said represent the most significant findings thus far in his career, are the most definitive to date showing that chromosomal DNA is accessible to and can be controlled by synthetic and natural molecules.
"With this information, one could easily turn on or off gene expression, as well as think about ways to correct genetic disease by changing mutant gene sequences back to normal," Dr. Corey said. "Those types of things now look a lot more feasible."
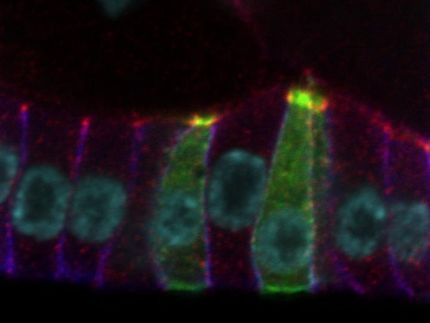
Genes are segments of DNA housed in the chromosomes in the nucleus of every cell. Genes carry instructions for making proteins, which in turn carry out all of life's functions. Faulty or mutated genes lead to malfunctioning proteins, which cause disease.
The information in a gene is not directly converted into proteins, but first is copied by special enzymes into many copies of messenger RNA, which then move out of the nucleus and into the body of the cell, where they go on to create a protein.
Current techniques for turning genes on or off focus on controlling the messenger RNA once it's already produced. But blocking all the copies of messenger RNA before they can make a protein within a cell is akin to using a bucket to catch all the streams of water coming out of a yard sprinkler before they can hit the ground.
While that's certainly possible, a more efficient way to staunch the streams of water would be to turn off the faucet. By targeting the chromosomal DNA directly, that's just what Dr. Corey and his colleagues accomplished.
The researchers targeted chromosomal DNA in two ways. First, they developed a synthetic molecule called a peptide nucleic acid, or PNA, which physically binds to DNA and blocks enzymes from copying, or transcribing, the DNA into messenger RNA.
More importantly, the researchers also employed RNA itself as a silencing agent. Previous work by other scientists had shown that RNA might be able to target chromosomal DNA, so once Dr. Corey and his team saw that PNAs were working, they decided to try RNA as well.
"The RNA is more important because it may reflect the body's own natural mechanism for controlling gene expression, while the PNAs are synthetic," Dr. Corey said.
"The experiments worked beautifully," he said. "It's hard to believe that this strategy would work so well if nature wasn't doing it already."
The researchers designed their RNA to match up with and target specific genes. "It's possible that the body is making the RNAs that we are using, and that will be an exciting topic for further research, to determine whether the human body or viruses and bacteria make RNA sequences like this to control gene expression," Dr. Corey said.
So far, the researchers have inhibited the expression of nine different genes in cancer cell cultures. Dr. Corey said it's not clear whether the RNA is actually binding to the DNA itself, as the PNAs do, but it's clear the effects are occurring at the DNA level.
Other UT Southwestern researchers involved in the studies were lead author on both papers Dr. Bethany Janowski, research assistant professor in pharmacology; Dr. Kunihiro Kaihatsu, former pharmacology postdoctoral research fellow; Dr. Kenneth Huffman, pharmacology postdoctoral research fellow; Jacob Schwartz, pharmacology research assistant; Rosalyn Ram, pharmacology research associate; Dr. Daniel Hardy, biochemistry postdoctoral researcher; David Shames, student research assistant; Dr. Carole Mendelson, professor of biochemistry; and Dr. John Minna, professor and director of the Nancy B. and Jake L. Hamon Center for Therapeutic Oncology Research and the W.A. "Tex" and Deborah Moncrief Jr. Center for Cancer Genetics.
The study was supported by the National Institutes of Health and The Welch Foundation.