Thermal Stability Technology Selection Guide for Biotherapeutic Drug Development

Guide to automated biologics stability testing for quality and regulatory support
Biologics or biotherapeutics, derived from living organisms, transform disease treatment with greater specificity and efficacy than traditional small-molecule drugs. Examples include vaccines and monoclonal antibodies (mAbs), rapidly growing due to their versatility. However, they have complex structures and require sophisticated characterization and manufacturing processes.
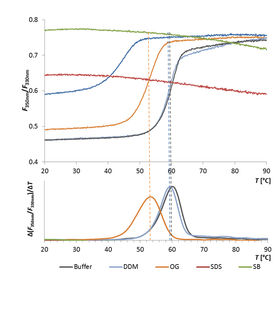
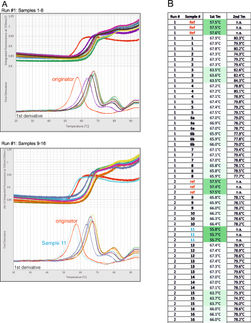
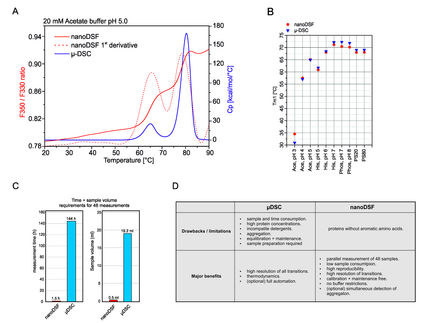
Thermal stability testing is mandatory, as regulations require stability studies to prove safety and efficacy throughout the shelf life, measuring likelihood of aggregation. Key methods include Differential Scanning Calorimetry (DSC) and Nano Differential Scanning Fluorimetry (DSF). DSC measures heat during thermal transitions like protein unfolding, while DSF is a fluorescence-based protein stability assay. DSC offers high precision but is slower and requires more sample; DSF offers high throughput but with less detailed data.
For those seeking DSC quality with DSF efficiency, Rapid-Screening DSC is ideal. This guide covers various thermal stability testing methods and their applications.
Download white paper now

Thermal Stability Technology Selection Guide for Biotherapeutic Drug Development
Guide to automated biologics stability testing for quality and regulatory support