Fertilizer and Fuel
Researchers elucidate how a nitrogen-fixing enzyme also produces hydrocarbons
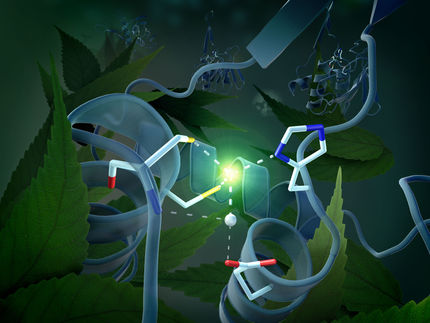
plants need nitrogen and carbon to grow. photosynthesis allows them to take in the latter directly from the air, but they have to procure nitrogen through their roots in the form of organic molecules like ammonia or urea. Even though nitrogen gas makes up approximately 80 percent of Earth’s atmosphere, the plant can only access it in a bound – or ‘fixed' – form. Farmers thus use fertilizers to provide their crops with nitrogen. The only living beings that can convert nitrogen from the air into usable molecules are microorganisms – for example nodule bacteria. They possess the enzyme nitrogenase, which combines nitrogen with hydrogen to form ammonium. In a new study, Prof. Dr. Oliver Einsle and Dr. Thomas Spatzal have not just contributed to the further elucidation of how this enzyme functions, but also described a unique mechanism it uses to produce hydrocarbons.
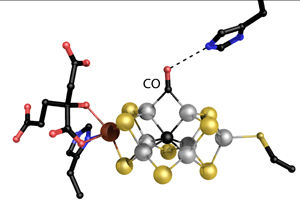
Einsle and his team developed this new crystal structure of the metal core of the nitrogenase. It demonstrates how carbon monoxide (CO) while it is binding to a hydrogen molecule, dislocates a sulfure atome.
Oliver Einsle
“We want to understand the reactions of nitrogenase in order to make the enzyme available for future biotechnological applications. At present, half of humankind can only be fed through the use of fertilizers in agriculture. This consumes roughly one percent of the world’s energy production,” explains Einsle. The researchers demonstrated for the first time how nitrogenase converts carbon monoxide. This leads to the production of molecules that resemble biofuels. “Thus, the enzyme is also interesting for sustainable energy production,” says Einsle.
Einsle is studying the microstructure of the heart of the enzyme: a large metal core called the iron molybdenum cofactor (FeMoco). Einsle, Spatzal, and Prof. Dr. Douglas Rees in Pasadena, USA, have obtained a crystal structure that shows how a carbon monoxide molecule (CO) binds to FeMoco. “There, it unexpectedly pushes away a sulfur atom that had previously occupied the same position in the metal core. For the first time, this allows us to draw inferences on how the core reacts with other molecules,” describes Einsle. The researchers published their findings in the journal Science.
“A chemical rearrangement of this kind has never been observed before in a biological system,” explains Einsle. It has been known since 2010 that CO, an inhibitor of nitrogenase, is slowly converted to hydrocarbons to a minimal extent. By applying CO gas to the enzyme during the nitrogenase reaction, the researchers found a binding site for CO and succeeded in documenting the rearrangement. Thus, in addition to the so-called “Haber-Bosch process of nitrogen fixation,” nitrogenase also stimulates a reaction corresponding to the “Fischer-Tropsch synthesis of hydrocarbons,” which can be used on a large scale to synthesize fuels, for instance from industrial waste gases. “The new structural analysis is the first ever description of the mechanism of this unusual reactivity,” says Einsle.
Original publication
Thomas Spatzal, Kathryn A. Perez, Oliver Einsle, James B. Howard, Douglas C. Rees (2014) Ligand binding to the FeMo-cofactor: Structures of CO-bound and reactivated nitrogenase. Science
Most read news
Original publication
Thomas Spatzal, Kathryn A. Perez, Oliver Einsle, James B. Howard, Douglas C. Rees (2014) Ligand binding to the FeMo-cofactor: Structures of CO-bound and reactivated nitrogenase. Science
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.