Targeted therapy identified for protein that protects and nourishes cancer
Scientists discover Skp2 inhibitor that blocks malignancy-promoting effects, stymies cancer stem cells and shrinks tumors in preclinical studies
Scientists at The University of Texas MD Anderson who identified a protein's dual role in cancer promotion have discovered a way to shut it down, opening a potential new avenue for cancer treatment.
Reporting this week in the journal Cell, the researchers describe the first compound that directly binds to and blocks Skp2, a protein they previously showed both turns off a cellular defense against cancer and switches on a cancer-feeding metabolic pathway.
"The beauty of this study is we identified an inhibitor and showed how it functions to block Skp2. Inhibitors often are discovered without an initial understanding of how they work," said co-senior author Hui-Kuan Lin, Ph.D., associate professor of Cellular and Molecular Oncology at MD Anderson.
Lin teamed with co-senior author Shuxing Zhang, Ph.D., assistant professor of Experimental Therapeutics and head of the Integrated Molecular Discovery Laboratory at MD Anderson, to identify and characterize the drug.
"There are many more chemical compounds available than there are estimated stars in the universe," Zhang said. "We have a database with 10 million compounds, but our prescreening analysis narrowed our computerized search to 120,000 and then further to find small-molecule candidates that inhibit Skp2."
Their inhibitor plugs critical binding sites on Skp2, preventing it from connecting to Skp1 to form a complex, which is the first step in its two cancer-promoting functions, Lin said.
Compound hits prostate, lung tumors
"This compound has a high degree of specificity – our tests in prostate and lung cancer show it preferentially targets the cancer cells but not the normal cells," Lin said. Steps remain to define the drug's potential off-target effects before it can advance to human clinical trials, Lin said.
The researchers also found that the inhibitor suppresses prostate cancer stem cells, which play a role in cancer initiation, progression and resistance to chemotherapy.
Normally, Skp2 E3 ligase binds to and tags other proteins with molecules called ubiquitins, which can serve as activation signals or as targets marking the protein for destruction. Skp2 is overexpressed in numerous cancers and plays a critical role in cell cycle progression leading to cell division, metabolism and dormancy, as well as cancer progression and metastasis.
Lin and colleagues previously showed that Skp2 promotes cancer by:
- Marking for destruction a cancer-stifling protein called p27 that renders cells senescent, or incapable of dividing.
- Firing up a signaling pathway that activates glucose metabolism (glycolysis), which cancer cells primarily rely upon to grow and survive.
They also showed that the glycolysis pathway contributes to Herceptin resistance and shorter survival among breast cancer patients whose tumors heavily express the HER2 protein.
Structural analysis identifies vulnerabilities
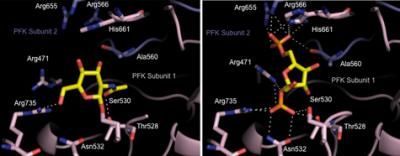
By analyzing the connection between Skp2 and Skp1, Zhang's group identified two pocket-like regions on Skp2 where the proteins connect.
Skp2 has been a logical target for cancer therapy, Zhang said, but presented two major obstacles.
Targeting protein-protein interactions is already difficult and Skp2 has a huge area where it interfaces with other proteins, making it hard to find one small molecule to completely block that surface.
"To begin such a search, to rationally design a drug, you must first understand the target's biology and then look at its structure and fully comprehend its complex interactions and how disrupting those will help treat the disease," Zhang said. "Once you understand those, you're ready to screen using computer models."
Virtual screening of the 120,000 compounds with a program developed by Zhang revealed 25 candidates that bind to either or both pockets. Additional analyses showed that Compound #25, also known as SZL-P1-41, effectively disrupted Skp1-Skp2 interactions.
Subsequent experiments showed Compound #25 suppresses Skp2-related tagging and destruction of the cell dormancy protein p27, restoring its expression in prostate cancer cells, and also stifles Skp2 signaling that activates the cancer-feeding glycolysis pathway.
Detailed experiments showed the drug's effect is achieved by binding specifically to one of the pockets on Skp2 to disrupt formation of the Skp2-Skp1 complex.
Inhibitor kills multiple types of cancer cells
Initial cell line experiments showed Compound #25 selectively destroyed prostate cancer cells with minimal effects on normal tissue. The drug's effects were confirmed in two lung cancer cell lines and in liver and osteocarcinoma cell lines.
Underlying mechanisms for the drug's effect on prostate cancer cells proved to be cellular senescence initiation and glycolysis suppression.
Recent research indicates that glycolysis is important to cancer stem cell formation and that senescence through telomere shortening restrains cancer stem cell growth.
Since Compound #25 blocks glycolysis and promotes senescence, the team tested its effect on prostate cancer stem cells.
Treatment stymies cancer stem cell formation, shrinks tumors
Treating prostate cancer cells with the compound reduced the population of cancer stem cells in a dose-dependent manner. The drug didn't work at all in cancer cells where Skp2 had been silenced.
Because cancer stem cells are a major cause of chemotherapy resistance, the researchers treated cell lines with Compound #25 and either of the chemotherapies doxorubicin or cyclophosphamide. Combining the new drug tripled the cancer cell growth inhibition of doxorubicin and doubled that of cyclophosphamide.
Finally, evasion of cellular senescence and promotion of glycolysis are hallmarks of cancer progression and drug resistance. The team tested its drug in mice with prostate and lung tumors. In both cases, treated mice had tumors about a quarter of the size of those in mice injected with a control agent.
Lin, Zhang and MD Anderson have filed for patent protection of this work.