Antisense Pharma Granted Patents on Medical Device for Application of Neurotherapeutics
Antisense Pharma GmbH has announced the granting of patents for an application system, which for the first time allows long-term and outpatient administration of therapeutic substances into brain tissue using the so-called Convection Enhanced Delivery (CED). The portable system has been specially developed for the treatment of brain tumor patients using Antisense Pharma‘s innovative drug trabedersen. Patents currently granted cover Europe, the USA, Canada, Japan and India, with more patents in other countries being filed
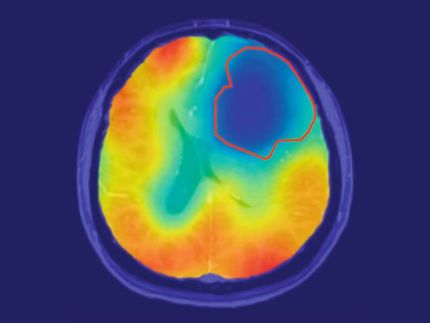
The two sides of the blood-brain barrier – causing 98% of potential neurotherapeutics to fail
There is a physiological diffusion barrier, which controls the exchange of substances between the circulating blood and the brain in order to protect the sensitive brain, ensuring a stable environment in the brain tissue. High molecular, charged or polar substances require specific transport systems in order to reach the brain – an effective protection, which however means that the blood-brain barrier blocks about 98% of potential neurotherapeutics.
“The difficulty of administering a therapeutic dose of a pharmacologically effective substance into the brain tissue is one of the main reasons for the currently very few therapy options we can offer to brain tumor patients “, says Dr. Karl-Hermann Schlingensiepen, Chief Executive Officer of Antisense Pharma. “There is only a very small number of substances capable to pass the blood-brain barrier so that they can be administered orally or by means of intravenous infusion“.
Convection Enhanced Delivery (CED) for optimum penetration of tissue
While an (intraventricular or intrathecal) injection or the placement of a drug carrier in the brain tissue circumvents the blood-brain barrier, however the regional diffusion of the drug is very limited. The concentration of the administered substance is therefore high in the direct vicinity of the place of injection, but it rapidly decreases with increasing distance. In contrast, the new application system by Antisense Pharma uses a special infusion technology. Convection Enhanced Delivery (CED) provides a high and uniform concentration of active substances in the brain tissue – even at a distance from the place of infusion. The substance is administered directly into the tumor or the surrounding brain tissue using a permanent, pressure-supported infusion using a special catheter/pump system. Since CED builds up a pressure gradient, a clearly higher and more homogeneous penetration volume can be achieved compared to conventional diffusion. The substance penetration depends on the tissue density, catheter diameter, flow rate, and others.
Intracranial use of CED – a high-precision technology
Originally developed at the National Institutes for Health (USA), the potential of the CED technology has now been documented by numerous animal studies, clinical studies and state-of-the-art imaging systems. 3,4,5,6 This technology offers new application options for new and also for already approved substances – but its clinical use requires a suitable application system: “A CED-based intracranial infusion is a challenge for the pump technology used. Our brain is enclosed by solid cranial bones. The available space tolerates only minor infusion volumes which have to be infused smoothly using a constant pressure “, Dr. Hubert Heinrichs, Chief Medical Officer at Antisense Pharma explains. This mode of administration has so far required the use of stationary application systems and the patients had to be hospitalized for the complete therapy, involving an increased risk of infection. With the development of the first portable, now patented CED application system, the German entrepreneurs are cutting edge: “The option of a CED-based treatment using a portable application system over weeks and months could well stimulate the development of urgently required neurotherapeutics “, Dr. Heinrichs takes a look ahead.
Trabedersen, the drug developed by Antisense Pharma to combat malignant brain tumors, is also not able to pass the blood-brain barrier. “Earlier examinations of brain tumor cells had already shown the very high efficacy of trabedersen“, Dr. Schlingensiepen remembers. “Due to the molecular structure, it was obvious that we would have to administer the substance directly into the brain for an extensive period of time. It is Antisense Pharma’s declared philosophy to allow critically ill people not only a longer life expectancy, but also a clearly improved quality of life. We have therefore developed in parallel to the compound also a very patient-friendly application system“.
The infusion system which has now been patented consists of carefully harmonized components: after the catheter has been stereotactically placed in the tumor, the drug is administered via an infusion line using an external, programmable, portable pump. The implantation of the catheter is a neurosurgical intervention, which is geared to the placement of a shunt for hydrocephalus patients where excessive brain liquid is diverted into the blood circulation. “The new application system uses the innovative approach so that a known and secure technology is used successfully in a completely new clinical context“, Dr. Heinrichs explains. The brain catheter is inserted through the skin to a port chamber which is usually placed in the chest muscle. The chamber is then connected to an external infusion line, which in turn is connected to a small, portable infusion pump that can easily be attached to the belt.