On-off Switch for Enzymes
Researchers at TU Graz have gained new insights into the functioning of a protein found in bacteria, whose enzymatic activity is activated by blue light.
Light affects living organisms in many different ways: for example, plants orient their growth direction towards the sun, while circadian rhythms in humans are controlled by daylight. These processes always involve photoreceptors, which are proteins that can sense different colours and intensities of light.
10,000-fold increase in enzymatic activity
Now, researchers at Graz University of Technology (TU Graz) have deciphered the function of a highly efficient photoreceptor. Their findings have been published in the journal Science Advances. The research team studied a diguanylate cyclase protein that is found in many bacteria. Its enzymatic function regulates the production of a central messenger substance that controls the way bacteria live. In darkness, the protein is almost completely inactive, but as soon as it is exposed to blue components of daylight, its enzymatic activity increases rapidly. “The protein’s enzymatic activity is about 10,000 times higher when it is exposed to light than it is in the dark," said Andreas Winkler, Head of the Photobiochemistry Working Group at TU Graz’s Institute of Biochemistry. In most photoreceptors, activity increases by a factor of between 5 and 50, resulting in more gradual changes in protein activity. “By contrast, the protein that we characterised reacts very strongly, so it actually works like an on-off switch,” Winkler explained. An efficient protein switch like this could be used in future to enhance and optimise optogenetic tools.
Protein stretches under blue light
The researchers have now unlocked the architecture and function of the protein switch. The protein consists of two functional parts: one is responsible for the perception of blue light, and the other for the actual enzymatic activity, serving as the catalyst for a chemical reaction. If it is exposed to blue light, the protein changes its structure. When it is inactive, the whole protein is in a compact form, but when it comes into contact with light, the protein stretches, connecting the previously separated enzymatic parts. Then, the protein produces specific messenger molecules which signal to the bacterium that environmental conditions are changing. If possible, the bacterium adapts to these new conditions. “An example of this is the formation of aggregates, known as biofilms, which make bacteria more resistant to environmental influences," Andreas Winkler explained.
Potential medical application
“I’m really excited that our research has generated valuable insights into the mechanism of this fascinating protein," commented Uršula Vide, first author of the study and a PhD student at the TU Graz Institute of Biochemistry. “Understanding the mechanism behind this light-activated enzyme switch opens the door to possible applications in a range of different disciplines.” One of these is in optogenetic treatment methods used in medicine. Drugs linked to a light-regulated protein switch could take effect at a precise time and only in a very limited area of the body, which would reduce potential side effects. A light-induced protein switch would also deliver benefits for research into cell biology, as this would enable targeted triggering of specific changes at the molecular level that could then be analysed more effectively. “But we are still a long way away from such practical applications of this particular switch,” Winkler pointed out. However, he believes that his team’s research has produced some important, fundamental insights.
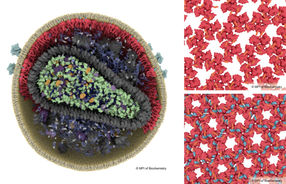
Three-dimensional model
For their experiments, the researchers did not isolate the protein from the original bacteria, but instead produced it in the laboratory with the help of genetic engineering. They used X-ray diffraction to analyse the molecular structure, which formed the basis for a three-dimensional model. Combined with supplementary experiments, this model allowed the researchers to draw inferences about the changes in the protein’s structure upon exposure to blue light, which translated into specific conclusions about the molecular function of the biological switch.