Why macrophages rest in healthy tissue
ETH scientists have shown that the immune system’s macrophages are regulated not only biochemically, but mechanically as well. This could explain why the cells are less active in healthy body tissue.
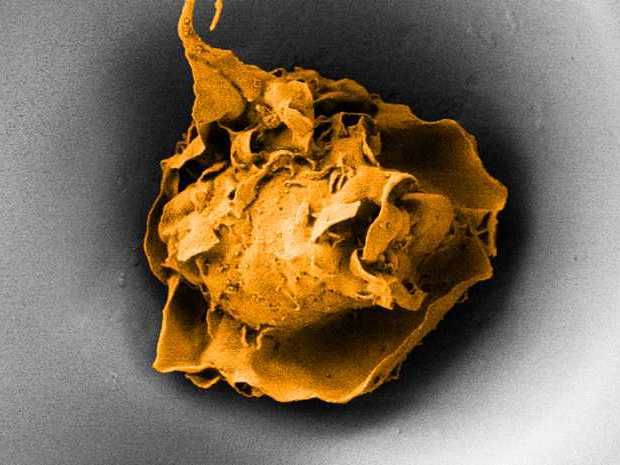
A macrophage in a pore measuring 20 micrometres across (electron microscope image).
ETH Zürich / Nikhil Jain und Isabel Gerber
Macrophages are a type of white blood cell. The term comes from the Greek for “big eater”, which describes one of the roles these cells play in our bodies: they detect and engulf pathogenic bacteria, triggering an inflammatory response that fights the infection. Macrophages are regulated by cytokines and other biochemical substances. These molecules stimulate macrophages to increase their activity when needed and to calm down once their job is done.
Viola Vogel, Professor in the Department of Health Science and Technology, and postdoc Nikhil Jain have now discovered another regulatory mechanism for pro-inflammatory macrophages: spatial information. When macrophages sit in the tissue between other cells and are constricted by them, their activity is lowered – even if upregulating stimuli are present.
No unnecessary inflammatory responses
“Not only do macrophages circulate in the blood, they can also be found in all the body’s tissues. They wait quietly, like guards, until they are needed,” Vogel explains. She says it’s very important for the body that tissue residing macrophages stay inactive, as they could otherwise trigger unnecessary inflammatory responses: “Macrophages don’t need to be activated until the tissue has been damaged.” However, the mechanisms that dampen pro-inflammatory macrophage activity in healthy tissues have so far not been fully understood.
By conducting cell culture experiments in the lab, the ETH scientists have been able to determine that macrophages are regulated both biochemically and mechanically. By placing macrophages into pore-like indentations, they could restrict the spreading of individual cells.
These experiments also allowed the researchers to unlock underpinning molecular mechanisms. The function of a macrophage is tied to its size: if it is activated, it grows by expanding its cytoskeleton. This releases factors that influence the activity of the genes. The macrophage, however, cannot grow if it is caged in by external barriers.
Understanding diseases and improving implants
The discovery that spatial factors steer the activation of macrophages has major implications for the understanding of various diseases. “Many age related diseases are associated with the latent production of pro-inflammatory cytokines as produced by insufficiently controlled macrophages,” Vogel says. This includes rheumatic diseases, atherosclerosis, obesity, cancer and several auto-immune diseases.
In addition, the discovery will spark new ideas how to structure the surfaces of implants so as to reduce inflammation. “We show that the structure of a material surface affects the macrophage response,” Vogel says. The next step could be to see if, for example, patients who receive implants with porous surfaces form less scar tissue around it, as suggested already by some previous observations.
























































