From Enzymes to MOFs
Advertisement
UK scientists have used dioxygenase enzymes to create enantiopure organic molecules, which in turn have been used to create chiral metal-organic frameworks. Dioxygenase enzymes were sourced from modified bacterial whole cells (mutants), isolated from the environment. These were then used as biocatalysts for the asymmetric cis-Dihydroxylation of azaarenes creating enantiopure bioproducts.
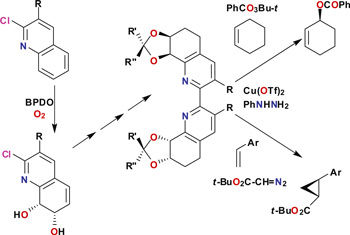
Derek Boyd and colleagues from Queens University Belfast used the dioxygenase enzymes to synthesise chiral 2,2'-bipyridines, and utilised these as homogeneous catalysts for the asymmetric oxidation of alkenes. 'Future developments will lie in the optimisation of the biotransformations by using new or improved biocatalysts' explains Boyd. 'The cis-dihydrodiol moiety has considerable synthetic versatility and has the potential to provide a much wider range of chiral ligands.'
Dioxygenase enzymes used to create single enantiomer products
The possibilities of this methodology have been evidenced further by Stuart James, Derek Boyd and colleagues from Queens University Belfast, Newcastle University, the University of Southampton and Durham University, where the researchers have synthesised 4,4'-bipyridines, and used these chiral building blocks to make chiral MOFs. 'Our paper' explains James 'describes a unique application of chemoenzymatic synthesis to provide well-behaved enantiopure organic building blocks to make chiral MOFs in a rational way. Stable, chiral versions of classical MOF building blocks, such as 4,4'-bipyridyl are highly desirable, but are not generally available. We recognised a unique opportunity to address this.'
Original articles: Derek Boyd et al., Chem. Commun., 2008.
Stuart L. James, Derek Boyd et al., Chem. Commun., 2008.

























































