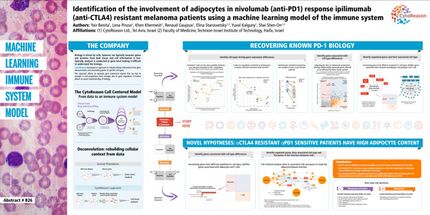
FDA grants Investigational New Drug status to Advanced Life Sciences' malignant melanoma compound
The food and Drug Administration has granted Investigational New Drug (IND) status to Advanced Life Sciences for the compound ALS-357, allowing the company to initiate clinical testing. Advanced Life Sciences will evaluate the safety and tolerability of the topical application of ALS-357 in clinical trials involving patients with in-transit metastatic disease involving the skin.
"Because the incidence of malignant melanoma is increasing in Western countries and the five-year survival rate for individuals diagnosed with this condition remains low, there is a very significant market need for an effective treatment," said Dr. Michael T. Flavin, CEO of Advanced Life Sciences. "We are excited about achieving this latest milestone in our drug development program and we look forward to commencing human clinical trials on ALS-357."
Metastatic melanoma, a cancer that has spread from its original site, is uniquely resistant to most currently available cancer drugs, and in many instances, may result in the death of the patient.
ALS-357 is a natural product, derived from birch bark, which has demonstrated anti-tumor activity against malignant melanoma.
According to the American Cancer Society, melanoma is the fifth most common cancer in men and sixth most common cancer in women. The Centers for Disease Control estimates 59,350 new cases of malignant melanoma in the United States in 2004 and 7,800 deaths. Melanoma is the most common cancer among people aged 25 to 29.
Other drugs in clinical development at Advanced Life Sciences include calanolide A, a naturally occurring anti-HIV agent currently entering Phase II clinical testing and ALS-886, a novel therapy for the treatment of Acute Respiratory Disease Syndrome (ARDS) that is currently in Phase I clinical trials.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.