ID Biomedical begins development of pandemic influenza vaccine
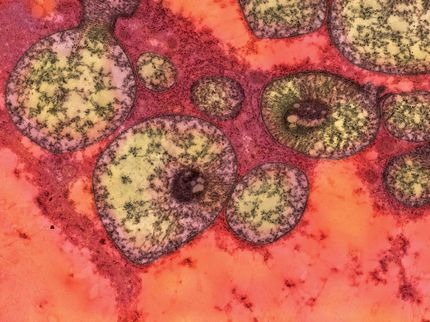
ID Biomedical Corporation announced that it has begun development of an experimental ("mock") vaccine against a strain of the influenza virus, H5N1, that experts believe could cause a deadly worldwide epidemic (pandemic) of influenza. The H5N1 strain is a new strain of influenza virus that humans have little or no resistance to and has resulted in high rates of illness and death. ID Biomedical's development efforts will concentrate on creating a vaccine using a genetically modified variant of the H5N1 strain.
Since January 2004, sporadic outbreaks of highly pathogenic bird (avian) influenza caused by an H5N1 influenza strain have occurred in South East Asia. Occasional transmission of the virus directly from birds to human has resulted in a high mortality rate in those infected. To date, all humans infected have contracted the virus directly from birds and there is no conclusive evidence of human to human transmission that could lead to an influenza pandemic.
ID Biomedical has obtained the genetically modified rH5N1 reference strain from the National Institute for Biological Standards and Control (NIBSC) in the U.K. NIBSC is one of the few World Health Organization (WHO) selected laboratories in the world, which conducts experimental work aimed at producing pandemic reference strains for potential vaccine production.
The reverse genetics technology used by NIBSC allows the rH5N1 strain to be grown in chicken eggs, an important source of influenza vaccine production. Typically, because of their virulence, pandemic avian strains kill eggs, therefore making it impossible to produce a vaccine from this source. The genetic modification of the virus also weakens its virulence so that it is no more dangerous than the influenza strains used by manufacturers during annual influenza production.
The initial objective of the Company's development program is to optimize the conditions under which the rH5N1 virus grows in eggs and to develop a virus seed bank for future vaccine production. The work will be conducted by ID Biomedical at its Quebec manufacturing facility. The second phase of this pandemic preparedness program is to produce a "mock" vaccine in sufficient quantities to conduct clinical trials. ID Biomedical is currently in discussions with the Canadian Government with regard to funding this important program.
Most read news
Organizations
Other news from the department research and development
These products might interest you

Systec H-Series by Systec
Safe, reproducible and validatable sterilization of liquids, solids and waste
Autoclaves with 65-1580 liters usable space, flexibly expandable for various applications

Whatman™ folded filter papers by Cytiva
Whatman folded filter papers
Convenient folded formats speed up your sample preparation

Get the life science industry in your inbox
From now on, don't miss a thing: Our newsletter for biotechnology, pharma and life sciences brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.