Geron Announces Publication Showing In Vivo Efficacy Of An Oncolytic Virus Containing The Telomerase Promoter
Advertisement
Menlo Park. Geron Corporationannounced the publication of research demonstrating selectivity, efficacy and lack of toxicity of an oncolytic virus containing the human telomerase promoter. The work was published in the August issue of Cancer Gene Therapy by scientists from Genetic Therapy, Inc. (an affiliate of Novartis AG) and Cell Genesys, Inc.
Oncolytic viruses are designed to treat cancer by employing the natural properties of lytic viruses-they invade cells, replicate inside the cell until it is destroyed, and then spread to adjacent cells where the process is repeated. In one class of oncolytic virus, the virus is engineered using a promoter that is preferentially active in tumor cells to control expression of a gene necessary for viral replication. The result is that the virus replicates in - and kills - cancer cells without damaging most other cells.
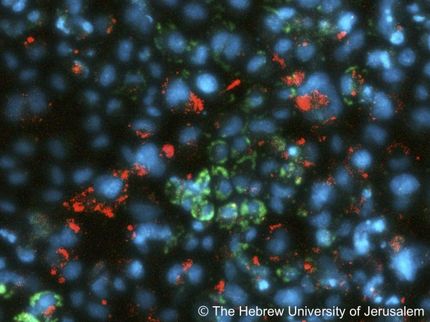
The new publication describes an oncolytic adenovirus (CG 4030) that contains two tumor-selective promoters, each driving a gene in the virus that is required for viral replication: the telomerase promoter (active in over 90% of all human cancers) driving the viral E4 gene and the E2F-1 promoter (active in tumors that have a defective Rb pathway, approximately 85% of all human cancers) driving the E1A gene. In vitro assays showed that the virus killed multiple human cancer cell lines while having little effect on normal cells, including cultures of primary liver cells. This virus was significantly less toxic in normal liver cells than an earlier version which did not contain the telomerase promoter.
The researchers administered the virus intravenously to mice and found it to be well tolerated, as well as less toxic than the earlier, single-E2F-1 promoter version. In mice bearing human liver and prostate tumors, the virus resulted in significant reduction in tumor growth. For instance, a single intravenous injection of the virus caused complete tumor regression in 80% of the animals bearing human prostate cancer tumors. Moreover, the combined administration of the virus and doxorubicin, a commonly used chemotherapeutic agent, to mice bearing human liver tumors resulted in significantly improved anti-tumor activity compared to either agent alone.
"These data show the dual-promoter oncolytic adenovirus to be a promising approach to treating cancer," said Thomas B. Okarma, Ph.D., M.D., Geron's president and chief executive officer. "The addition of the telomerase promoter reduces toxicity to normal cells, especially the liver, while preserving broad anti-tumor activity. The telomerase enzyme must be turned on for cancer cells to replicate indefinitely. Because telomerase is expressed in virtually all types of cancer cells and few other cell types, the telomerase promoter can make an oncolytic virus very selective, and hence less toxic. This virus and others like it could be developed for treatment of a wide range of cancers, including metastatic disease, alone or in combination with chemotherapy."
Geron granted a nonexclusive license to Genetic Therapy, Inc., an affiliate of Novartis AG, to develop oncolytic viruses that employ the telomerase promoter to drive selective viral replication in cancer cells. Cell Genesys acquired Genetic Therapy's rights under that license in its acquisition of Genetic Therapy's oncolytic virus assets.
Geron's proprietary telomerase technology is also the foundation for two other anti-cancer development programs. Geron's telomerase inhibitors, GRN163 and GRN163L, drugs that block the telomerase enzyme and send cancer cells into crisis, are in preclinical testing. Geron's cancer vaccine, which induces an immune response specific to telomerase-bearing cancer cells, is in a Phase I/II clinical trial for hormone-refractory metastatic prostate cancer at Duke University Medical Center.