Provocative prions may protect yeast cells from stress
prions have a notorious reputation. They cause neurodegenerative disease, namely mad cow/Creutzfeld-Jakob disease. And the way these protein particles propagate – getting other proteins to join the pile – can seem insidious.
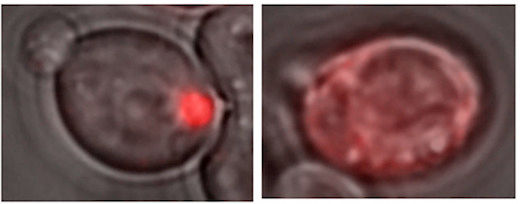
A glowing red clump can be detected in yeast cells containing a Lsb2 prion (left), because Lsb2 is hooked up to a red fluorescent protein. In other cells lacking prion activity (right), the Lsb2 fusion protein is diffuse.
Emory University
Yet prion formation could represent a protective response to stress, research from Emory University School of Medicine and Georgia Tech suggests.
A yeast protein called Lsb2, which can trigger prion formation by other proteins, actually forms a "metastable" prion itself in response to elevated temperatures, the scientists report.
Higher temperatures cause proteins to unfold; this is a major stress for yeast cells as well as animal cells, and triggers a "heat shock" response. Prion formation could be an attempt by cells to impose order upon an otherwise chaotic jumble of misfolded proteins, the scientists propose.
"What we found suggests that Lsb2 could be the regulator of a broader prion-forming response to stress," says Keith Wilkinson, PhD, professor of biochemistry at Emory University School of Medicine.
The scientists call the Lsb2 prion metastable because it is maintained in a fraction of cells after they return to normal conditions but is lost in other cells. Lsb2 is a short-lived, unstable protein, and mutations that keep it around longer increase the stability of the prions. If the Lsb2 protein is hooked up to a red fluorescent protein, a glowing red clump can be detected in prion-containing cells (see figure).
The result was the result of collaboration between Wilkinson, Emory colleague Tatiana Chernova, PhD, assistant professor of biochemistry, and the laboratory of Yury Chernoff, PhD in Georgia Tech’s School of Biology.
Other investigators studying yeast prions have been finding examples of how they may help cells adapt to a changing environment . The Emory/Georgia Tech findings are consistent with this idea, Chernova says. She notes that the Lsb2 protein’s acquisition of the ability to form prions coincides in evolution with when the baker’s variety of yeast (Saccharomyces), in which prion-forming Lsb2 is found, became adapted to higher temperatures.
"We don't know if these prions are adaptive," she says. "Maybe some are, while others could be pathogenic, but the whole ability of converting misfolded proteins into ordered prions instead of amorphous toxic agglomerates could be protective, and this process could be adaptive."
Understanding how and why prions form could illuminate Alzheimer’s disease research; the behavior of the toxic protein fragment beta-amyloid, central in Alzheimer’s, sometimes resembles that of prions. The authors note that the yeast protein Lsb2 has some sequence similarity to a human protein called Grb2, known to interact with APP, the precursor of amyloid-beta.
Original publication
Tatiana A. Chernova, Denis A. Kiktev, Andrey V. Romanyuk, John R. Shanks, Oskar Laur, Moiez Ali, Abheek Ghosh, Dami Kim, Zhen Yang, Maggie Mang, Yury O. Chernoff, Keith D. Wilkinson; "Yeast Short-Lived Actin-Associated Protein Forms a Metastable Prion in Response to Thermal Stress"; Cell Reports; 2016
Original publication
Tatiana A. Chernova, Denis A. Kiktev, Andrey V. Romanyuk, John R. Shanks, Oskar Laur, Moiez Ali, Abheek Ghosh, Dami Kim, Zhen Yang, Maggie Mang, Yury O. Chernoff, Keith D. Wilkinson; "Yeast Short-Lived Actin-Associated Protein Forms a Metastable Prion in Response to Thermal Stress"; Cell Reports; 2016
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Translational Genomics Research Institute (TGEN) - Phoenix, USA

M&A in chemicals and life sciences slowed down by global lockdowns - Race for COVID-19 vaccine

Verder Group acquires ERWEKA - With this acquisition, Verder Scientific expands its portfolio to include dissolution and tablet testing equipment for the pharmaceutical and life science sectors

AlgoNomics NV - Gent, Belgium
Proteros Achieves Key Milestone in Janssen Lead Discovery Collaboration
GO Therapeutics and Roche collaborate in new glycotargeting bispecific cancer treatment
Millipore Expands BioPharma CRO Services in Europe