Biosensor measures signaling molecules within cilia
Scientists can measure the dynamics of signaling molecules in subcellular compartments
Advertisement
Scientists of the Research Center caesar in Bonn, an Institute of the Max Planck Society, developed a new biosensor, which allows to measure nanomolar levels of the second messenger cAMP. The sensor makes it possible to study cAMP signaling with high precision, even in subcellular compartments. Using this new biosensor, the scientists of the Minerva Max Planck Research Group “Molecular Physiology“ headed by Dagmar Wachten and of the Department “Molecular Sensory Systems” headed by Benjamin Kaupp revealed how the production of cAMP is regulated in the flagella of sperm cells from mice.
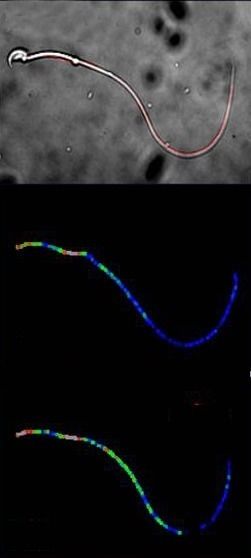
Above, an image of a single mouse sperm acquired with a light microscope is shown. Within this image, the flagellum is marked red. The middle image displays a normal and the lower image an increased cAMP concentration along the flagellum. Whereby blue corresponds to a low and red to a high cAMP concentration. A comparison of the two images demonstrates: cAMP concentration changes differ significantly in different areas of the flagellum.
© Forschungszentrum caesar
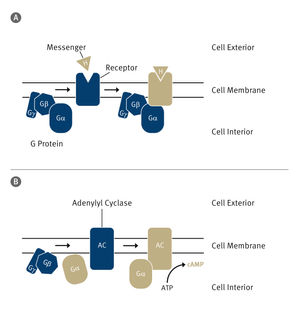
Cells can change the way they grow, move, or develop in response to stimuli from their environment. This information is first detected at the surface of the cell and then translated into an intracellular response by signaling molecules known as “second messengers”. A molecule called cAMP is a well-known second messenger that is involved in many different signaling pathways.
Some cells have hair-like structures called cilia or flagella on their surface, which have been proposed to function as antennae for extracellular stimuli. Cilia are membrane protrusions that come in two different flavors - they can be motile or immotile. A special case of a motile cilium is the flagellum with the most prominent example being the sperm flagellum.
The second messenger cAMP plays an essential role in making motile cilia move, but it is challenging to analyze, how the levels of this molecule change over time in these structures. The levels of cAMP in live cells can only be measured using fluorescent biosensors. Introducing these biosensors into subcellular compartments is difficult and so far, the sensors have not been sensitive enough to respond to low levels of cAMP. Furthermore, it is difficult to measure cAMP activity inside such tiny structures using these biosensors.
The team of scientists created a new cAMP biosensor that has several unique features. The sensor is based on FRET (Förster resonance energy transfer) technology. FRET is a mechanism describing energy transfer between two light-sensitive molecules. In the new created biosensor, the amount of energy tranfer depends on the distance and orientation between two light-sensitive molecules. Upon binding to cAMP, a structural rearrangement increases the distance of the two molecules and thereby decreases the amount of energy transferred between them.
Most importantly, the sensor can respond to very low levels of cAMP, making it more sensitive than previous biosensors. The caesar scientists tested this new biosensor in the flagella of sperm cells from mice, which revealed how the production of cAMP is regulated in the flagellum. The new biosensor also showed that different parts of the flagellum can have different cAMP dynamics.
In the future, this new biosensor could be used to study cAMP in other structures and compartments within cells.
Original publication
Mukherjee, S., Jansen, V., Jikeli ,J. F., Hamzeh, H., Alvarez, L., Dombrowski, M., Balbach, M., Strünker, T., Seifert, R., Kaupp U. B. & Wachten, D.; "A novel biosensor to study cAMP dynamics in cilia and flagella"; eLife, 2016