Structure-based design used as tool for engineering deimmunized biotherapeutics
Dartmouth investigators engineer drug candidates for improved compatibility with immune system
Advertisement
In the first experimental use of algorithms that employ structure-based molecular modeling to optimize deimmunized drug candidates, Karl Griswold, PhD, and co-investigator Christopher Bailey-Kellogg, PhD of Dartmouth College complement their prior sequence-based deimmunizing algorithms and expand the tool kit of protein engineering technologies to use in next generation drug development. Their paper, "Protein Deimmunization via Structure-based Design Enables Efficient Epitope Deletion at High Mutational Loads," was published in Biotechnology and Bioengineering.
NCCC
"This work is part of our larger collaborative initiative to develop performance-enhanced protein drugs that are invisible to the human immune system," explained Griswold. "Biotherapeutics offer potent treatment options for a wide range of diseases but, due to their biological origins, these powerful therapies can elicit detrimental immune responses in humans."
Development of biotherapeutic agents is a time-consuming and costly endeavor, and there exists a substantial risk that deleterious immunogenicity issues will undermine otherwise promising drug candidates late in the development process. While methods for identifying immunogenic hotspots, or epitopes, are evolving rapidly, technologies to redesign the hotspots while maintaining biotherapeutic activity and stability are far less developed.
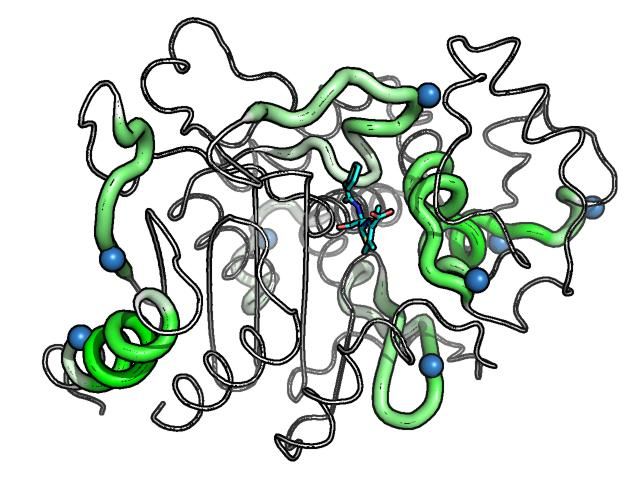
This study used P99 betalactamase, a component of Antibody Directed Enzyme Prodrug Therapy, to show that structure-based deimmunization resulted in highly-active and stable biotherapeutic designs that were different from those generated with earlier sequence-based algorithms. In particular, the structure-based designs remodeled a putative immunogenic hotspot that was not readily addressed with other methods.
"We demonstrated that integrating molecular modeling into deimmunizing algorithms enables simultaneous redesign of numerous immunogenic hotspots distributed throughout a protein target," Bailey-Kellogg said. "These results suggest that even the most immunogenic drug candidates might be engineered for improved compatibility with the human immune system."
Dartmouth's team of Griswold and Bailey-Kellogg are turning their attention to advanced assays and methodologies to better assess the immunogenic potential of their deimmunized drug candidates. These methods should yield clinically relevant data showing the extent to which they have mitigated the immunogenicity risk of target proteins in the drug candidates.