Small change for a big improvement – halogen bonds and drug discovery
Halogen bonding has been applied in crystal engineering, materials research, and nanotechnology for some time. Scientists from the Heidelberg Institute for Theoretical Studies (HITS) and the Czech Academy of Science in Prague have now developed a new tool to use halogen bonds for drug discovery applications.
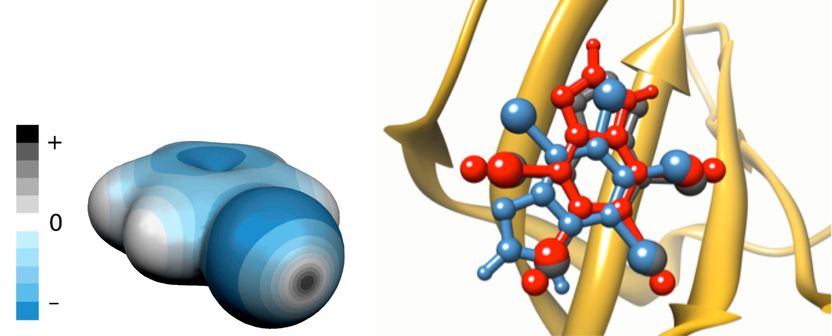
Left panel: the charge distribution around the bromobenzene molecule. The regions of negative electrostatic potential are in blue, positive regions in grey. The grey disc in the forefront represents the sigma-hole. Right panel: the overlay of the predicted binding poses of K17 inhibitor of casein kinase 2 (PDB code 2OXY) with (red) and without (blue) explicit sigma-holes (ESH) and comparison with the crystal structure (grey).
HITS
Halogen chemistry has been exploited by medicinal chemists for nearly 70 years. To date, halogens were regarded useful for optimization of so-called ADMET properties – they improve oral absorption and facilitate crossing biological barriers by prospective drugs, they are useful for filling small hydrophobic cavities present in many protein targets, and they prolong lifetime of the drug. In short: They make compounds of interest more drug-like. However, direct interactions mediated by halogen atoms have been much ignored in pre-clinical drug development.
Recently, scientists from Heidelberg and Prague, working in quantum chemistry and structure-based drug design, have developed a new tool for the usage of halogen bonds for computational medicinal chemistry and drug discovery applications. The study, led by Dr. Agnieszka Bronowska from the Heidelberg Institute for Theoretical Studies (HITS) and conducted in cooperation with scientists from the Czech Academy of Sciences.
Most halogens - except fluorine - have unique properties which allow them to stabilize direct interaction between prospective drugs and their protein targets. These properties are of quantum-chemical origin; namely, the anisotropy of charge distribution around the halogen atom, when it is bound to an electron-withdrawing substrate. Unexpectedly, despite of being negatively charged, halogens have regions which remain positively charged. These regions, called sigma-holes, are responsible for the directional and stabilizing character of halogen bonding with other electronegative atoms, such as oxygen or nitrogen.
Overlooking sigma-holes leads to errors in predictions of structure and energetics of drug-protein complexes and thus to failure in drug development.
By approximating the positively charged sigma-hole with a massless, charged pseudo-atom (denoted as explicit sigma-hole or ESH), Agnieszka Bronowska and her colleagues incorporated a quantum-chemical effect into faster (and much less accurate) computational methods applicable to structure-based drug design. “We tested nearly a hundred complexes between medicinally relevant proteins and halogenated molecules”, Bronowska says. “The results showed significant improvement in the description of such complexes upon introduction of ESH.”
The new method is already used by research groups in the Czech Republic, in the United Kingdom and in the U.S. for designing novel compounds to treat chemotherapy-resistant cancers, infectious diseases, and Alzheimer’s disease.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.