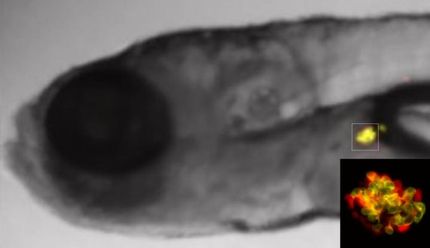
Anchoring proteins influence glucose metabolism and insulin release
Advertisement
Scientists from the United States and Sweden have discovered a new control point that could be important as a drug target for the treatment of diabetes and other metabolic diseases. A-kinase anchoring proteins or AKAPs are known to influence the spatial distribution of kinases within the cell, crucial enzymes that control important molecular events related to the regulation of glucose levels in the blood. In a new study published in The EMBO Journal, the team of researchers led by Simon Hinke and John Scott reveal for the first time that AKAPs influence the levels of glucose in the body by coordinating the spatial positioning of Phosphatases, naturally occurring enzymes that counteract the effects of kinase enzymes.
“Our discovery that anchored enzymes contribute to the regulation of cellular events that underlie diabetes may help us to move more rapidly toward new therapies to control this increasingly prevalent metabolic disease,” commented John Scott, Edwin G. Krebs–Hilma Speights Professor of Pharmacology at the University of Washington School of Medicine, Seattle and an investigator of the Howard Hughes Medical Institute. “The observation that AKAP 150 functions by coordinating phosphatase activity in the cell reveals a new role for these anchoring proteins in the control of glucose metabolism and related metabolic disorders. It also suggests that new drugs that interfere with the role of anchoring proteins are possible therapeutic interventions to treat chronic diseases such as diabetes.”
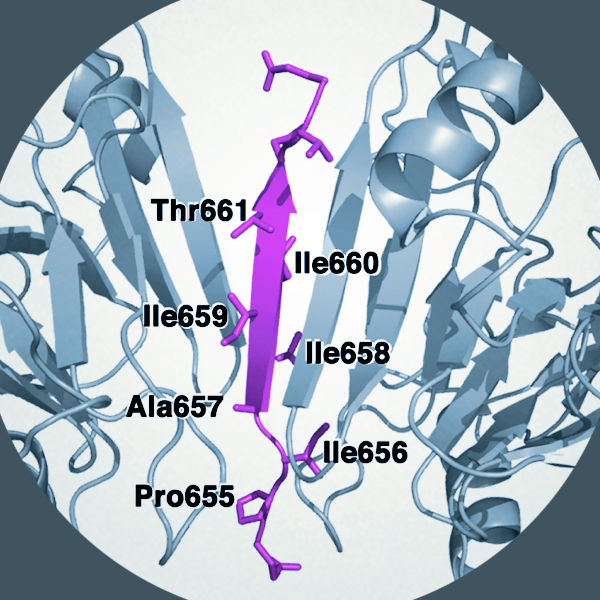
The researchers used imaging techniques as well as genetic modification of isolated insulin-secreting cells and whole mice to investigate the impact of anchoring proteins on glucose metabolism and insulin release. Mice that lacked the gene for the AKAP150 anchoring protein produced less insulin from beta cells in the islets of Langerhans. However, they coped better with limited amounts of hormone due to increased sensitivity to insulin in the target tissues (skeletal muscle). The scientists showed that these effects are due to a seven-amino-acid sequence in the anchor protein that directly interacts with the surface of the phosphatase enzyme.
The release of insulin is the main way in which the levels of glucose are controlled in the body. If it is possible to develop drugs that target the region where anchoring proteins specifically interact with phosphatase enzymes it is feasible that insulin sensitivity could be improved in selected tissues such as skeletal muscle. This would represent a valuable new molecular control point that might offer clinical benefits for diabetics, individuals with other metabolic disorders and patients who are being treated with immunosuppressive drugs following organ transplantation.