Observed live for the first time: photocatalysts switch off a virus
Whitening agent titanium dioxide kills viruses – now researchers see it at work at the nanoscale
Advertisement
An international research team led by DESY scientists has been able to observe for the first time how the whitening agent titanium dioxide inactivates viruses. Using various advanced methods, including measurements at the synchrotron light source PETRA III, they were able to precisely reproduce the inactivation process and understand the atomic details by computational simulations. DESY scientist Heshmat Noei and her team have now published the results of their analyses in the journal ACS Applied Materials & Interfaces.
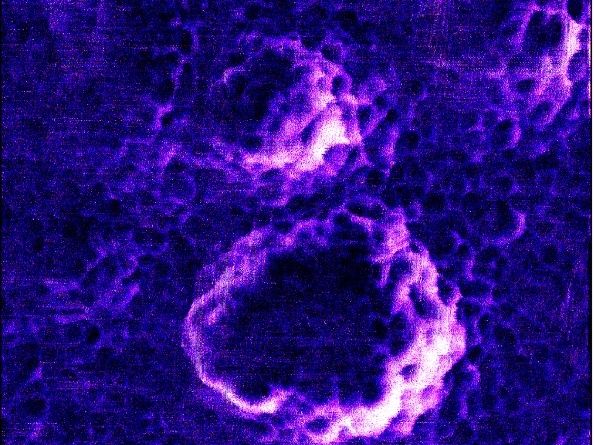
Atomic force microscopy demonstrates the virus morphology (spherical particles in image) and spike proteins on virus structure.
Mona Kohantorabi, DESY NanoLab
Titanium dioxide (TiO2) is a bright white substance that is often used in wall paints or sunscreens. A relatively thrilling property of this compound is that its photoactivity enables it to destroy viruses that come into contact with it. The research team has now been able to decipher the exact process that takes place on the surface of the titanium dioxide.
The researchers used virus-like particles (VLPs) for their investigations, which mimic real SARS-CoV-2 viruses but lack genetic materials (RNA). “These VLPs have the great advantage that they have similar structural proteins and morphology to real virus, however, cannot replicate and can therefore be handled safely without the need of specialized laboratories,” says research leader Noei from the DESY NanoLab. In this case, it was a Covid (SARS-CoV-2) VLP prepared by the Helmholtz Munich Research Centre in the groups of Wolfgang Hammerschmidt and Reinhard Zeidler with its characteristic spike proteins on its surface that was available to the researchers.
Using atomic force microscopy of the DESY NanoLab, the group observed that the VLPs retain their shape and size when they first come into contact with the titanium dioxide surface. At the PETRA III beamline P03, they were then able to observe what happens to the virus. “We found that while the structure of the virus remained intact under nitrogen atmosphere and in the dark, the VLPs on the titanium dioxide surface were denatured and went under morphological changes as soon as ultraviolet light hit the sample in air. The air acts as a source of oxygen. The UV light triggers an oxidation process in the amino acids blocks of the VLP´s protein, which is facilitated by the titanium dioxide as a photocatalyst,” explains the paper’s first author Mona Kohantorabi. This increases the size of the particle and ultimately destroys it. The researchers´ interpretation is corroborated by theoretical modelling through quantum mechanical calculations which were performed in collaboration with the group of Cristiana Di Valentin at the University of Milano-Bicocca in Italy. The researchers assume that the denaturation of proteins and the rupture of the virus membrane happen during the adsorption and oxidation process on the surface of TiO2. “Using the live measurements at P03, PETRA III, we have for the first time comprehensively understood the morphological changes of the VLPs, the structural proteins and the viral by-products simultaneously,” says Noei. “These findings are vital for the development and optimization of self-cleaning materials, as they highlight the mechanisms that ensure the thorough inactivation of enveloped viruses like SARS-CoV-2.”
Original publication
Mona Kohantorabi, Aldo Ugolotti, Benedikt Sochor, Johannes Roessler, Michael Wagstaffe, Alexander Meinhardt, E. Erik Beck, Daniel Silvan Dolling,... Reinhard Zeidler, Stephan V. Roth, Cristiana Di Valentin, Andreas Stierle, Heshmat Noei; "Light-Induced Transformation of Virus-Like Particles on TiO2"; ACS Applied Materials & Interfaces, Volume 16, 2024-7-3





















































