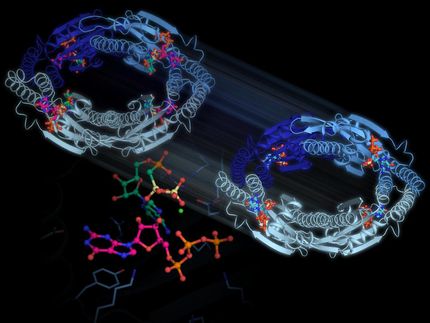
Using PETRA III to watch the disabling of a penicillin killer
Scientists observe in detail the binding and formation of covalent bonds of an inhibitor to a bacterial enzyme that disables common antibiotics
Advertisement
antibiotic resistance is a major and particularly in recent years a growing challenge in medicine. Scientists around the world are searching for new and efficient compounds to treat bacterial infections, especially infections caused by multi-resistant bacteria. A research collaboration of scientists from DESY; University Medical Center Eppendorf (UKE) in Hamburg, and Universität Hamburg performed time-resolved diffraction experiments at PETRA III to observe at near atomic resolution and at the millisecond timescale the inhibition of a bacterial enzyme that nullifies a common class of antibiotics, the β-lactams. The results have been published in Nature Communications Chemistry.
Among antibiotics, beta-lactams are the classics. Penicillin, the first commercially produced antibiotic, and the related derivatives from penicillin belong to this class of pharmaceuticals. At the beginning of the 21st Century, half of the antibiotics used worldwide applied were beta-lactams. However, even since the beginning of the use of penicillin, bacteria have evolved defences against antibiotics. One of the defences is an enzyme called beta-lactamase. Like a molecular pair of scissors, beta-lactamase cuts the central ring of the beta-lactam molecule and disables its antibiotic properties – allowing the bacteria to keep living.
Worldwide and for the last 20 years scientists worldwide have been searching for a way to disable beta-lactamase in an effort to directly combat antibiotic resistance. Until now, most of the candidate beta-lactamase inhibitors that have been examined have been organic compounds that mimic penicillin, allowing the inhibitor to enter the enzyme’s active site and block it. However, today’s bacteria can potentially resist these molecules after around one or two years as well. A different avenue of research has taken to using far more basic molecules to block the active site of the enzyme.
“There are new boric acid-based beta-lactamase inhibitors, and they are really potent,” says Andreas Prester, the first author of the PETRA III study and a postdoc at UKE. “For example, boron-containing compounds and drugs were developed to be used for the treatment for multiple myeloma, a form of blood cancer.” In terms of pilot investigations and a drug re-purposing approach, the research collaboration identified the potential of boron-based compounds to inhibit beta-lactamases as well. “Since then we’ve studied these inhibitors in more detail, as well as their potential to inhibit beta-lactamses,” Prester adds.
Prester and his colleagues, Markus Perbandt from Universität Hamburg; Winfried Hinrichts, an emeritus professor from the University of Greifswald; and Christian Betzel, a professor at Universität Hamburg who led the research have been among those examining the inhibition caused by boric acid in detail. Using the European XFEL and PETRA III, they examined how the boric acid binds to the enzyme. At PETRA III, the team around DESY lead scientist Henry Chapman helped assemble an experiment at the beamline P11 that used a mechanism that could show at atomic resolution, like a movie, the progress of boric acid binding, in this case, to the amino acid serine within the active site. “It’s a relatively stable bond, and the boric acid then blocks the ability of the enzyme to interact with the antibiotic,” says Prester.
The setup provided by Chapman’s group could show the timescale of the bonding using a method called mix and inject serial crystallography, in which the compound is mixed with tiny protein crystals at a defined point in time before probing with X-rays. The research collaboration found that boric acid alone binds to the enzyme in around 100 milliseconds. In a follow-up approach, the team also used the same method to observe how the organic molecule glycerol binds to the serine-boric-acid complex, in order to see how a more complex boric acid structure might bind to the enzyme. The boric acid bound to the active site via glycerol occurred at a similar timescale as the binding of boric acid to the site alone. “The information obtained is an initial stepping stone to later designing a drug that can inhibit the beta-lactamase and block the resistance to the antibiotic”, says Dominik Oberthür, a leading scientist at the Center for Free Electron Laser Science at DESY who was part of the research team.
“The type of compounds we applied and corresponding chemical reactions we observed time resolved are very far from the penicillin-like inhibitors, which we know encounter a high rate of resistance from the bacteria,” says Betzel, who is a member of the Excellence Cluster "CUI: Advanced Imaging of Matter". “Boric acid is a very small molecule in comparison. While the efficiency is comparatively low to some inhibitor compounds, the bacteria are highly unlikely to develop resistance to boric acid in the near or even distant future.” Additionally, should the boric acid become dislodged from the enzyme, allowing it to function again and disable the antibiotic, another boric acid molecule – or even the same one – can easily swoop in and once more block the active site, owing to its small size and stability.
Oberthür says the next steps are to further examine the behaviour of the boric acid in environments more similar to the human body, while also making steps towards medicinal chemistry. “It appears that boric acid is also effective on the metal-containing enzymes,” Prester adds. With that knowledge, penicillin and other beta-lactam antibiotics could still remain in humanity’s medicinal arsenal.
Original publication
Andreas Prester, Markus Perbandt, Marina Galchenkova, Dominik Oberthuer, Nadine Werner, Alessandra Henkel, Julia Maracke, Oleksandr Yefanov, Johanna Hakanpää, Guillaume Pompidor, Jan Meyer, Henry Chapman, Martin Aepfelbacher, Winfried Hinrichs, Holger Rohde, Christian Betzel; "Time-resolved crystallography of boric acid binding to the active site serine of the β-lactamase CTX-M-14 and subsequent 1,2-diol esterification"; Communications Chemistry, Volume 7, 2024-7-5