Awakening «dormant» cells to fight cancer
A team from the UNIGE and the HUG has succeeded in identifying a drug-targetable mechanism whose blockage reduces the capacity of melanoma cells to adapt and resist to treatment
The advent of small-molecule targeted therapies, a decade ago, revolutionized the treatment of metastatic melanoma, provided that the tumors carry the mutations to respond to these treatments. However, despite a remarkable initial response that can be seen in a majority of patients, most of them will undergo relapse even after spectacular initial responses. These relapses are due to “dormant” persistent cells, unresponsive to treatment. A team from the University of Geneva (UNIGE) and the University Hospitals of Geneva (HUG) has shown that these cells under-express a protein called HuR. By deciphering the mechanism of this insufficient expression and by targeting it with an enzyme inhibitor, this team has succeeded in reducing the therapeutic resistance of all melanoma cells. These results, published in Biochemical and Biophysical Research Communications, open new therapeutic avenues against metastatic melanoma and other types of solid cancers.
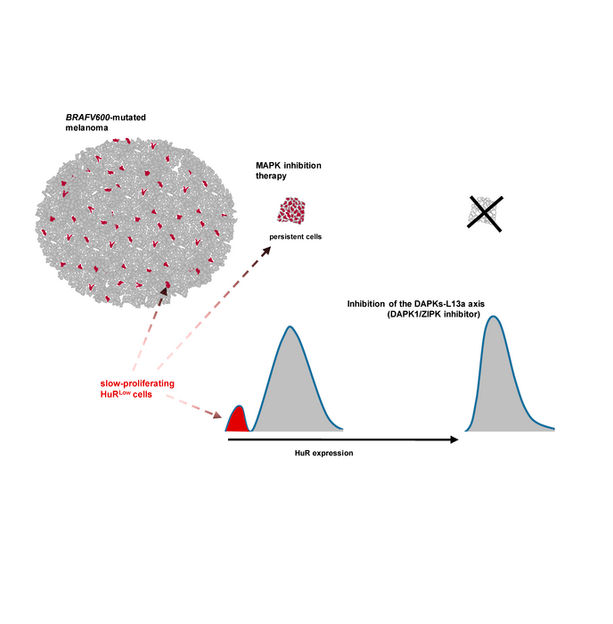
Caption Targeted inhibition of the DAPKs (Death-associated protein kinase 1 and 3) reduces the proportion of slow-proliferating/senescent-like HuRLow cells and the adaptive response of the whole melanoma-cell population to BRAF inhibition.
UNIGE / Rastine Merat
Melanoma is one of the most dangerous skin cancers. Potentially very aggressive, it develops from melanocytes, the cells responsible for skin pigmentation. The initial tumor can be superficial with a good prognosis upon removal, it can also be deeper and become metastatic, i.e. migrate to other organs in the body.
For the last ten years, thanks to the advent of the so-called small-molecule targeted therapies - drugs that inhibit a precise mechanism within the tumor to fight it - half of the metastatic melanomas that carry a genetic signature making them sensitive to these drugs, can be treated effectively, sometimes even be eradicated. “However, despite such spectacular initial responses, 80% of patients will suffer recurrences, and these recurrences will often occur in the same initially affected sites”, explains Rastine Merat, researcher in the Department of Medicine at the UNIGE Faculty of Medicine and head of the Onco-dermatology Unit at the HUG.
A protein regulating cell division is involved
This phenomenon is called ‘‘adaptive resistance’’: certain cancer cells adapt to the drugs used to fight them and lead to a resurgence of the disease. This happens even when the metastases - and therefore the cells that make these tumors - seem to have completely disappeared. “This is explained by the persistence, after treatment, of small residues of so-called ‘dormant’ malignant cells that conventional radiology tools are unable to detect,” says Rastine Merat. “The particularity of these cells, in addition to being invisible, is that they proliferate slowly. This characteristic helps the cells to escape therapy, even during the initial treatment.”
Previously conducted research has shown that in slow-proliferating cells, a protein that among other things regulates the expression of many genes that control cell division - the HuR protein - is insufficiently expressed. This is in contrast with rapidly proliferating cells in which this protein is highly expressed. In a research work published in 2019, Rastine Merat and his team had established the link between the insufficient expression of this protein and the melanoma cells’ capacity to resist to a targeted therapy. In their recent research, they have uncovered a specific mechanism involved in the insufficient expression of this protein in the “dormant” cells that can be targeted with drugs.
Inhibiting enzymes to prevent recurrence
“In the cells, messenger RNAs play a central role in protein production. In the minority of cells in which HuR is insufficiently expressed, we found that the messenger RNAs of HuR were trapped by some other proteins. This is at least one of the mechanisms that causes HuR insufficient expression.” By using a chemical compound to inhibit two kinases - enzymes - involved in this mechanism, the UNIGE team managed to prevent HuR insufficient expression, reducing the ability of all melanoma cells to resist to treatment.
“The great difficulty in carrying out this work was to work on this type of cells, which are difficult to detect and analyze because of their small number and the fact that the state of insufficient expression of HuR protein is dynamic and reversible at any time for any cells; i.e. at any time the same cells can start to proliferate and flip to a state of high expression of this protein”, explains the researcher. To do this, “we paradoxically overexpressed this protein in melanoma cells. This allowed us to make the mechanisms at play more easily detectable.” This discovery opens new perspectives in the treatment of melanoma, but not only. “Melanoma is a model cancer: if we understand it, we can understand many other types of solid cancers,” explains Rastine Merat.
For the researcher and his team, “the next step will be to encourage the pharmaceutical industry to optimize the inhibitors of the identified kinases, - to improve their stability and bioavailability - which is something pharmaceutical drug companies know how to do nowadays in a very systematic way, at least for this type of target”, concludes Rastine Merat.