"Make two out of one" - Division of Artificial Cells
Scientists uncover a novel and generic mechanism for the division of artificial cells into two daughter cells
The success of life on earth is based on the amazing ability of living cells to divide themselves into two daughter cells. During such a division process, the outer cell membrane has to undergo a series of morphological transformations that ultimately lead to membrane fission. Scientists at the Max Planck Institute of Colloids and Interfaces, Potsdam, and at the Max Planck Institute for Polymer Research, Mainz, have now achieved unprecedented control over these shape transformations and the resulting division process by anchoring low densities of proteins to the artificial cell membranes.
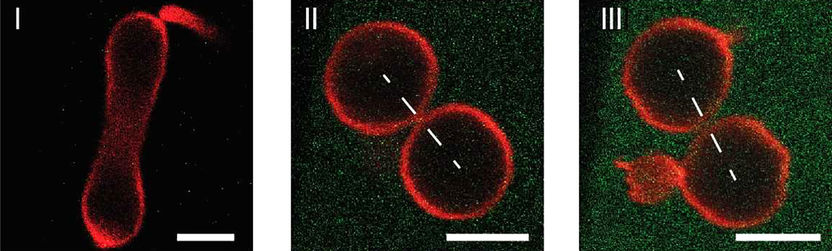
Division process of an artificial cell (red), which is controlled by the protein concentration (green) in the outer aqueous solution: (I) Low protein concentrations induce a prolate or dumbbell shape of the cell with a wide membrane neck. A further increase in the protein concentration leads to a closed membrane neck in (II) and to a curvature-induced constriction force that cleaves this neck in (III), thereby creating two separate daughter cells.
© Max-Planck-Institut für Kolloid- und Grenzflächenforschung/Jan Steinkühler
All living organisms on earth are built up from individual cells. Furthermore, the proliferation and growth of these organisms is based on the ability of each cell to divide into two daughter cells. During the division process, the cell membrane, which provides the outer boundary of the cell, has to undergo a series of morphological transformations that ultimately lead to the fission of the cell membrane. To control this process, today’s cells rely on highly specialized protein complexes, which are driven by ATP hydrolysis. It turns out, however, that controlled division can be achieved in a much simpler way, as researchers at the Max Planck Institute of Colloids and Interfaces, Potsdam, and at the Max Planck Institute for Polymer Research, Mainz, have recently demonstrated for artificial cells. These cells are provided by giant lipid vesicles, which have the size of a typical animal cell and are bounded by a single lipid membrane, which provides a robust and stable barrier between the interior and exterior aqueous solution. This compartmentalization is a crucial feature of cell membranes as well.
In addition, vesicle and cell membranes have essentially the same molecular architecture and consist of molecular bilayers with two molecular leaflets that define the two sides of the membranes: the inner leaflet is exposed to the interior, the outer leaflet to the exterior solution. On the one hand, artificial cells with a wide membrane neck remain stable for days and weeks. On the other hand, as soon as the neck has closed down the membrane generates a constriction force onto this neck that cleaves the neck and divides the artificial cell into two daughter cells.
Constriction forces generated by membrane asymmetry
In addition to demonstrating the division of artificial cells, the researchers around Reinhard Lipowsky also identified the novel mechanism, by which this constriction force can be controlled in a systematic manner. To do this, they designed membranes whose inner and outer leaflets differ in their molecular composition by exposing the outer leaflets to a variable concentration of protein. This asymmetry between the two leaflets generates a preferred or spontaneous curvature that determines the shape of the artificial cells. Furthermore, once a closed membrane neck has been formed, the spontaneous curvature generates a local constriction force that leads to the division of these cells. Thus, quite surprisingly, the complete division of the artificial cells is driven by the mechanical properties of the membranes: the force that divides the membrane neck arises directly from the asymmetry of the bilayer membranes.
Versatile module for synthetic biology
In this way, a simple and generic mechanism for the division of artificial cells has been identified. This mechanism does not depend on the precise nature of the molecular interactions that generate the bilayer asymmetry and the associated spontaneous curvature, as has been explicitly demonstrated by using different types of proteins. Furthermore, the used density of the membrane-bound proteins was rather low which leaves ample space for other proteins to be accommodated on the artificial cell membranes. Therefore, the membrane-protein systems introduced here provide a promising and versatile module for the bottom-up approach to synthetic biology. Finally, the division process of artificial cells described here also sheds new light on cell division in vivo. Even though all modern cells seem to rely on a complex protein machinery, our cellular ancestors may have used much simpler mechanisms for their division as Jan Steinkühler, the first author of the study, explains: “Certain bacteria can also divide without the known protein machinery. It has already been speculated that membrane mechanics might play an important role in the latter division processes. Our study demonstrates that mechanically controlled cell division is indeed possible."