How cells handle a sticky, toxic, but absolutely essential molecule
A new study shows that a protein known for its role in metabolism also helps deliver heme where it's needed
Advertisement
Do you enjoy breathing air? You should spare a thought once in a while for heme, an iron-containing molecule essential to all organisms engaged in an air-breathing lifestyle. Heme molecules are most famously part of hemoglobin, the oxygen-transporting protein in blood, but they are also components of numerous other proteins involved in gas transport and fundamental chemistry in cells. On its own, heme is toxic and reactive, but when slotted correctly into certain proteins, it's absolutely essential.
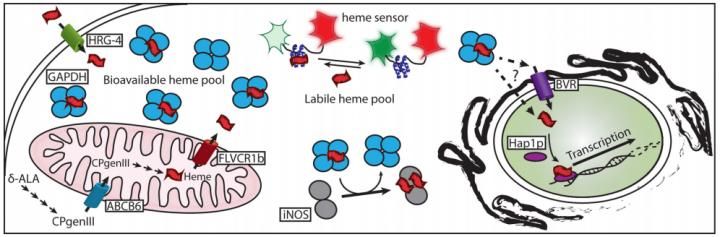
A recent paper describes how heme is chaperoned to its target proteins.
Dennis Stuehr, Cleveland Clinic
Until recently, a basic mystery about heme remained unsolved: How does it get from mitochondria, where it is made, to the proteins in other parts of the cell where it is needed?
A team of researchers at the Lerner Research Institute of the Cleveland Clinic has now solved this long-standing puzzle by identifying the protein that "chaperones" free heme in cells by binding to it, keeping it from doing damage to the cell until it's delivered where it's needed. The findings are published in the Journal of Biological Chemistry .
Dennis Stuehr, the investigator at Cleveland Clinic who oversaw the new study, had been interested in the mystery of the unknown heme chaperone for years. "It was surprising that really almost nothing was known," Stuehr said. "In the literature, it looks like everyone just turned the lights off and went home."
Bit by bit, Stuehr's team has been piecing together the biochemistry of free heme. The first step was finding out simply which proteins heme can bind to. Then, they needed to experiment to see which of the proteins that heme sticks to actually help it get to its final destination.
"Heme's kind of sticky; it binds to many things," said Elizabeth Sweeny, the postdoctoral fellow who was one of the co-leaders of the new study. "This (study) was the first time we found a protein that not only binds heme, and binds a lot of it, but is also required for delivery to downstream heme protein targets."
The new study uses several lines of evidence to implicate an unexpected player as heme's chaperone: glyceraldehyde 3-phosphate dehydrogenase, or GAPDH. GAPDH is an enzyme involved in breaking down sugar in cells. It's a commonplace, unglamorous component of the cell's basic metabolism, so much so that laboratory scientists mainly use it as a basic control in studies of other proteins.
"GAPDH is such a ridiculous candidate," Stuehr said. "But there's been this emerging story that GAPDH isn't just this boring glycolytic enzyme that's in every cell; it has these other roles in cell biology. And heme delivery is one of these new roles."
GAPDH may not be the only protein involved in chaperoning heme, Stuehr adds, and research on more details of how heme is delivered is ongoing.
"Our finding answers a cell biology question that's been around for some time, regarding the delivery mechanism of this essential biomolecule," Stuehr said. "Now we can think about and explore how disruption of this delivery process might actually contribute to a number of diseases, (like) anemias, asthma and more."