NanoTemper Technologies
Predicting the Long-Term Stability of Biologics
Analysis of Formulation-Dependent Colloidal and Conformational Stability of Monoclonal Antibodies
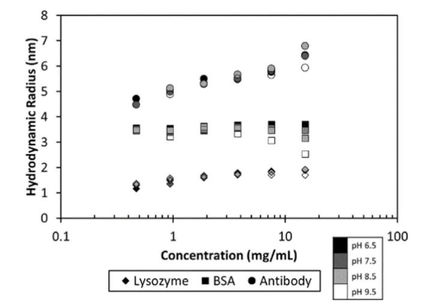
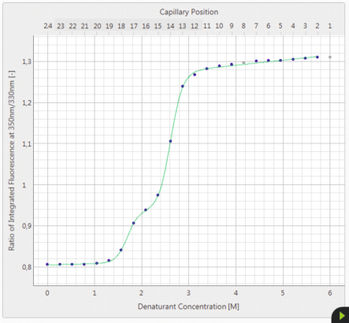
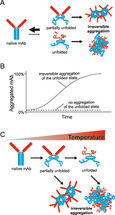
The growing number of biological drugs such as monoclonal antibodies (mAbs), as well as the wealth of heterogeneity between mAb variants requires a thorough development process to maximize mAbs compliance with regulation. Therefore, biophysical analytical methods are required already at early stages of the development process to guide and streamline further antibody processing and to predict antibody developability. In this case study, we demonstrate how the Prometheus NT.48 can be used to predict long-term mAb stability in a formulation screen by simultaneous quantification of both, conformational and colloidal stability of biologicals in thermal gradients.
Advertisement
White Paper classification
See the theme worlds for related content
Topic world Antibodies
Antibodies are specialized molecules of our immune system that can specifically recognize and neutralize pathogens or foreign substances. Antibody research in biotech and pharma has recognized this natural defense potential and is working intensively to make it therapeutically useful. From monoclonal antibodies used against cancer or autoimmune diseases to antibody-drug conjugates that specifically transport drugs to disease cells - the possibilities are enormous

Topic world Antibodies
Antibodies are specialized molecules of our immune system that can specifically recognize and neutralize pathogens or foreign substances. Antibody research in biotech and pharma has recognized this natural defense potential and is working intensively to make it therapeutically useful. From monoclonal antibodies used against cancer or autoimmune diseases to antibody-drug conjugates that specifically transport drugs to disease cells - the possibilities are enormous