Achieving Method Modernization with the New Liquid Chromatographic Gradient Allowances
Waters S.A.S.

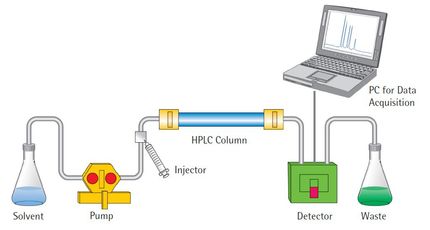
The extent to which the various parameters of a chromatographic test may be adjusted without fundamentally modifying the pharmacopeial analytical procedures is defined in U.S. Pharmacopeia (USP) General Chapter <621> Chromatography. In this application brief, we combine the gradient method adjustments described in this chapter with the Alliance™ iS HPLC System to achieve both column dimension and system modernization for the USP monograph separation of antiviral drug, abacavir sulfate.
Download white paper now

Achieving Method Modernization with the New Liquid Chromatographic Gradient Allowances