Measurement of secondary structure directly in formulation without interference from excipients

Highly sensitive measurements that overcome limitations of traditional spectroscopic tools
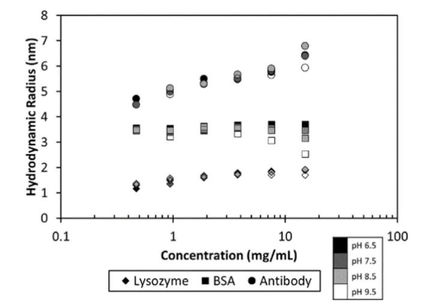
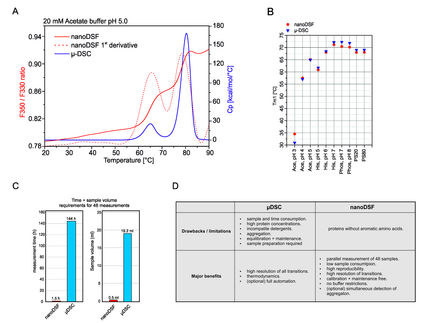
In this study, MMS was used to characterize the buffer-induced structural differences of lysozyme, a well-characterized alpha-helix-rich protein, in water and three common formulation buffers: phosphate buffer (PB), phosphate-buffered saline (PBS), and Tris buffer. Absorption spectra in the amide I region were automatically acquired and processed to calculate the percentages of higher order structures (HOS) and overall structural similarities between samples under all prepared conditions. The results showed that the enzyme exhibited different structural changes in the different buffers and that these changes could be quantified to make decisions about buffer selection to support ideal activity.
Download white paper now

Measurement of secondary structure directly in formulation without interference from excipients
Highly sensitive measurements that overcome limitations of traditional spectroscopic tools