Novel aggregation-specific fluorogen monitors prefibrillar protein aggregation by fluorescence polarisation (FP)
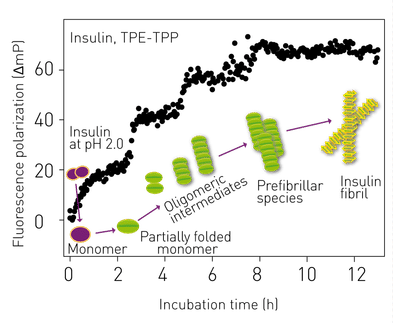
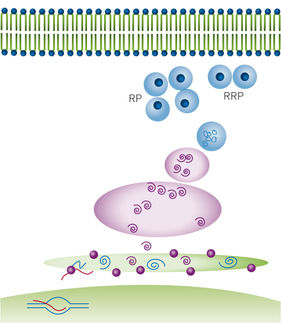
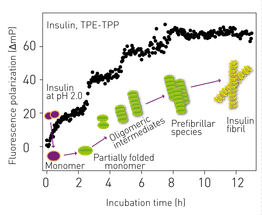
Highly ordered protein aggregates, termed amyloid fibrils, are associated with a broad range of diseases, many of which are neurodegenerative, for example, Alzheimer’s and Parkinson’s. The transition from soluble, functional protein to insoluble amyloid fibril occurs via a complex process involving the initial generation of highly dynamic early stage aggregates or prefibrillar species. Prefibrillar species (dimers, tetramers etc.) are proposed to play a key role in the cytotoxicity of amyloid fibrils. Therefore, novel probes that have broad applicability in the detection of prefibrillar species of amyloidogenic proteins are actively being sought.
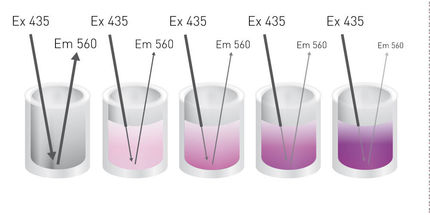
Recently, a novel broad-spectrum fluorescent probe was presented: (bis(triphenylphosphonium) tetraphenylethene (TPE-TPP)), with emission characteristics that are eminently suited to monitoring prefibrillar aggregation of various protein species using various fluorescence techniques. It is shown that TPE-TPP-based FP measurements can monitor the fibrillar assembly of a variety of amyloid fibril-forming proteins in situ.
Download white paper now
Novel aggregation-specific fluorogen monitors prefibrillar protein aggregation by fluorescence polarisation (FP)
White Paper classification
White papers on related topics
Products on related topics
Manufacturers of similar products
See the theme worlds for related content
Topic world Protein analytics
Protein analytics provides a deep insight into these complex macromolecules, their structure, function and interactions. It is essential for discovering and developing biopharmaceuticals, understanding disease mechanisms, and identifying therapeutic targets. Techniques such as mass spectrometry, Western blot and immunoassays allow researchers to characterize proteins at the molecular level, determine their concentration and identify possible modifications.

Topic world Protein analytics
Protein analytics provides a deep insight into these complex macromolecules, their structure, function and interactions. It is essential for discovering and developing biopharmaceuticals, understanding disease mechanisms, and identifying therapeutic targets. Techniques such as mass spectrometry, Western blot and immunoassays allow researchers to characterize proteins at the molecular level, determine their concentration and identify possible modifications.