Phase III development of Antisoma's ASA404 in lung cancer extended to Japan
Antisoma plc announced that ATTRACT-1, the Novartis phase III trial evaluating ASA404 as a first-line treatment for non-small cell lung cancer, is now enrolling patients in Japan. ATTRACT-1 has been enrolling patients in a variety of other countries since it began in April 2008. Extension of the trial to Japan follows the successful completion of a phase I study evaluating the safety of ASA404 in Japanese lung cancer patients.
Glyn Edwards, Antisoma's CEO, said "We're pleased that Japanese lung cancer patients can now participate in this key phase III trial of ASA404. This is an important step towards a potential application to market the drug in Japan."
ASA404 (DMXAA) is a small-molecule Tumour-Vascular Disrupting Agent (Tumour-VDA) which selectively disrupts tumour blood vessels, generating tumour death (necrosis) due to the resulting lack of blood flow in the tumour. The drug was discovered by Professors Bruce Baguley and William Denny and their teams at the Auckland Cancer Society Research Centre, University of Auckland, New Zealand. It was in-licensed by Antisoma from Cancer Research Ventures Limited (now Cancer Research Technology), the development and commercialisation company of the Cancer Research Campaign (now Cancer Research UK), in August 2001.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Transgene Receives FDA Fast Track Status for TG4010 for Treatment of Non-Small Cell Lung Cancer

A synthetic antibiotic may help turn the tide against drug-resistant pathogens - "This isn't just a cool new molecule, it's a validation of a novel approach to drug discovery"
Dräger AG Invests in Digital Health Startup GWA Hygiene - Digital hygiene solutions for hospitals
List_of_potato_diseases

Genetic Engineering without Unwanted Side-Effects Helps Fight Parasites - A live vaccine for toxoplasmosis

Going viral: Insights on Zika
Cell Biosciences Signs Agreement to Acquire Alpha Innotech
Findings on pollution damage to human airways could yield new therapies

New infection mechanism in coronavirus discovered - Results provide starting point for development of antiviral therapies
How obesity drives colon cancer in mice
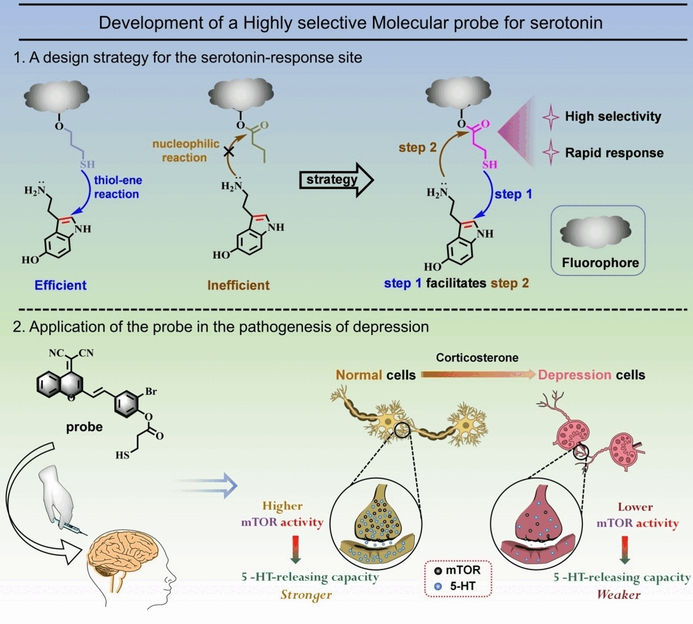
Tracking Depression - Molecular probe specifically detects serotonin in fluorescence imaging