Hollow gold nanospheres show promise for biomedical and other applications
Advertisement
A new metal nanostructure developed by researchers at the University of California, Santa Cruz, has already shown promise in cancer therapy studies and could be used for chemical and biological sensors and other applications as well.
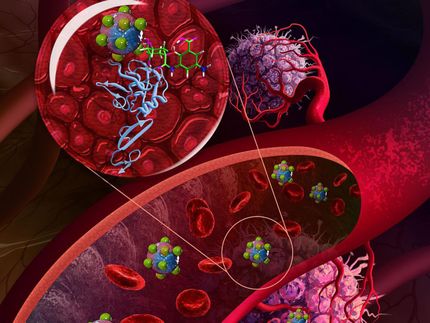
The hollow gold nanospheres developed in the laboratory of Jin Zhang, a professor of chemistry and biochemistry at UCSC, have a unique set of properties, including strong, narrow, and tunable absorption of light. Zhang is collaborating with researchers at the University of Texas M. D. Anderson Cancer Center, who have used the new nanostructures to target tumors for photothermal cancer therapy.
"What makes this structure special is the combination of the spherical shape, the small size, and the strong absorption in visible and near infrared light," Zhang said. "The absorption is not only strong, it is also narrow and tunable. All of these properties are important for cancer treatment."
Zhang's lab is able to control the synthesis of the hollow gold nanospheres to produce particles with consistent size and optical properties. The hollow particles can be made in sizes ranging from 20 to 70 nanometers in diameter, which is an ideal range for biological applications that require particles to be incorporated into living cells. The optical properties can be tuned by varying the particle size and wall thickness.
In the cancer studies, led by Chun Li of the M. D. Anderson Cancer Center, researchers attached a short peptide to the nanospheres that enabled the particles to bind to tumor cells. After injecting the nanospheres into mice with melanoma, the researchers irradiated the animals' tumors with near-infrared light from a laser, heating the gold nanospheres and selectively killing the cancer cells to which the particles were bound.
Cancer therapy was not the goal, however, when Zhang's lab began working several years ago on the synthesis and characterization of hollow gold nanospheres. Zhang has studied a wide range of metal nanostructures to optimize their properties for surface-enhanced Raman scattering (SERS). SERS is a powerful optical technique that can be used for sensitive detection of biological molecules and other applications.
Adam Schwartzberg, then a graduate student in Zhang's lab at UCSC, initially set out to reproduce work reported by Chinese researchers in 2005. In the process, he perfected the synthesis of the hollow gold nanospheres, then demonstrated and characterized their SERS activity.
"This process is able to produce SERS-active nanoparticles that are significantly smaller than traditional nanoparticle structures used for SERS, providing a sensor element that can be more easily incorporated into cells for localized intracellular measurements," Schwartzberg, now at UC Berkeley, reported in a 2006 paper published in Analytical Chemistry.
The collaboration with Li began when Zhang heard him speak at a conference about using solid nanoparticles for photothermal cancer therapy. Zhang immediately saw the advantages of the hollow gold nanospheres for this technique. Li uses near-infrared light in the procedure because it provides good tissue penetration. But the solid gold nanoparticles he was using do not absorb near-infrared light efficiently. Zhang told Li he could synthesize hollow gold nanospheres that absorb light most efficiently at precisely the wavelength (800 nanometers) emitted by Li's near-infrared laser.
"The heat that kills the cancer cells depends on light absorption by the metal nanoparticles, so more efficient absorption of the light is better," Zhang said. "The hollow gold nanospheres were 50 times more effective than solid gold nanoparticles for light absorption in the near-infrared."
Zhang's group has been exploring other nanostructures that can be synthesized using the same techniques. For example, graduate student Tammy Olson has designed hollow double-nanoshell structures of gold and silver, which show enhanced SERS activities compared to the hollow gold nanospheres.
The ability to tune the optical properties of the hollow nanospheres makes them highly versatile, Zhang said. "It is a unique structure that offers true advantages over other nanostructures, so it has a lot of potential," he said.
Original Publication: Clinical Cancer Research, February 1, 2009