Novel Diagnostics Methods Driving the European Cancer IVD Market towards Early Detection and Prevention
Early detection is the key focus of cancer diagnostics. Traditional testing methods such as immunoassay testing are facing fierce competition from evolving segments such as nucleic acid amplification technology (NAT) and immunohistochemistry testing. The ability to prove the clinical utility and practical use of the test within a laboratory is one of the essential requirements to be successful in this market. A new analysis from Frost & Sullivan, European Cancer IVD Market, finds that the market earned revenues of $623.5 million in 2008 and estimates this to reach $1.5 billion in 2015.
“Intense research for the development of new cancer therapies is increasing the demand for tests to guide physicians in the selection of appropriate treatments,” notes Frost & Sullivan Research Analyst Gayathry Ramachandran. “Repeated test requests, especially for prostrate specific antigen (PSA) will boost market revenues.”
The increased prevalence of cancer has resulted in the development of novel technologies that support the effective diagnosis of cancer. Rising awareness, paralleled by the mounting use of cancer screening tests will promote the growth of the European cancer IVD market over the long term.
The importance of molecular tests has been elucidated by the success of the human genome project. This has allowed the rapid proliferation of specific nucleic acid tests for the detection of tumour markers and this trend holds strong potential for the growth of the market.
“The human genome project has encouraged the cancer genome project, where high throughput techniques are developed to detect the mutated genes responsible for cancer,” explains Ramachandran. “Sizeable funding for cancer disease research programmes is also fuelling the development of cancer diagnostic tests.”
However, most cancer markers are not indicative of the progression of the disease or its incidence. In spite of tremendous strides in knowledge, the details of the biochemical and bio-cellular nature of cancer are still poorly understood.
“The validation of new tests is mandatory and challenges market participants to obtain certain regulatory approvals,” cautions Ramachandran. “Validation is a problem in multiplexing assays because the establishment of equivalency is difficult.”
The future of the cancer IVD market lies in the efforts and initiatives taken by governments to encourage R&D into companion diagnostics and predictive assays. This will determine the future of the market.
At the same time, alliances with pharmaceutical companies will mutually benefit both diagnostics and pharmaceutical companies, while accelerating the development of innovative tests and targeted drugs.
If you are interested in a virtual brochure, which provides manufacturers, end users and other industry participants with an overview of the European cancer in vitro diagnostic (IVD) market, then send an e-mail to Katja Feick, using the 'Contact' button below.
Most read news
Topics
Organizations
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
See the theme worlds for related content
Last viewed contents

Taking a cue from spider glue to create new materials
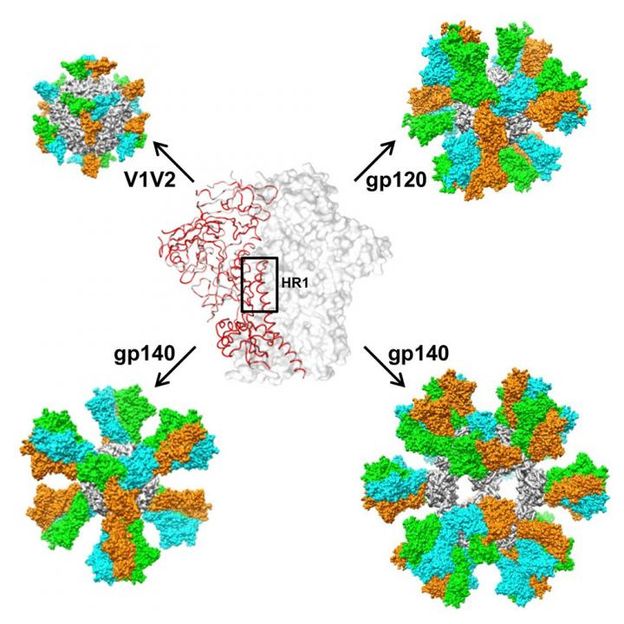
Scientists stabilize HIV structure, design potential AIDS vaccine candidates
New model finds HIV acute phase infectivity may be lower than previously estimated
LION bioscience AG erweitert Vorstand nach erfolgreich abgeschlossenem Erwerb der SYGNIS Bioscience GmbH & Co. KG
Chronic fatigue syndrome linked to imbalanced microbiome

Blueprint for blueberry improvement: genetic and epigenetic discoveries

New Bacterial Toxins Against Resistant Insect Pests - Scientists from the USA, Mexico, China, and Germany have developed Bt toxins for the management of resistance in European corn borer and other crop pests
In lab tests, new therapy slows spread of deadly brain tumor cells