Bavarian Nordic reports successful safety data from Phase II study with IMVAMUNE
Bavarian Nordic has completed a clinical safety report from a large Phase II study with IMVAMUNE® in HIV infected subjects that confirms the excellent safety profile of IMVAMUNE®. Within the next few days the safety report of this study will be submitted to the FDA and this will trigger a USD 25 million milestone payment under the RFP-3 contract. The clinical safety report constitutes a major part of the data package that will be used to potentially support the use of IMVAMUNE® in a declared emergency.
In support of using IMVAMUNE® as a smallpox vaccine in individuals otherwise contraindicated to receive conventional vaccinia vaccines, Bavarian Nordic has performed a large Phase II study in HIV infected subjects with CD4 counts between 200 and 750 cells/µl to compare the safety to healthy subjects.
The safety report from this study, which represents an essential part of the EUA data package, includes safety data from over 300 HIV infected and 86 healthy subjects, all of whom had no history of prior smallpox vaccination. The low number of adverse events confirmed the favourable safety profile of IMVAMUNE® in vaccinia-naïve HIV infected subjects with varying degrees of immune suppression. Indeed, there was no difference in adverse events between the healthy and HIV infected subjects, even in the most immune compromised patients (CD4 counts >= 200-350 cells/µl). IMVAMUNE® has now been tested in more than 2,200 people in 11 completed or on-going clinical studies, which includes a large proportion (more than 750) of immune-compromised people i.e. HIV infected or diagnosed with Atopic Dermatitis (AD) who are excluded from vaccination with traditional smallpox vaccines.
The complete data set from this trial is expected to be reported in the second half of 2009. The final report will include data on immunogenicity as well as long-term (6-month) safety information and will contain data from subjects enrolled in an additional study arm funded by the NIH under RFP-2 (HIV infected subjects with a history of previous exposure to a conventional smallpox vaccine).
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Genetic key discovered to dramatically increase yields and improve taste of hybrid tomato plants
The_Cancer_Center_at_the_University_of_Minnesota
Chloralose
Impression_(dental)
Medivir announces new studies in phase III program for TMC435 - Study in previous non-responder Hepatitis C genotype-1 infected patients
Amyotrophic_lateral_sclerosis
Ferriman-Gallwey_score
GE Healthcare completes acquisition of Whatman plc
Osmotic_demyelination_syndrome
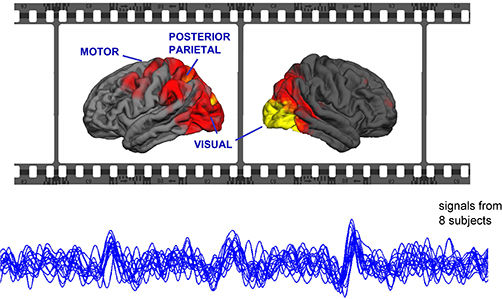
Movies synchronize brains - When we watch a movie, our brains react to it immediately in a way similar to other people's brains.
Innovative genetic and cellular techniques help identify multiple disease targets