Stanford Scientists Pinpoint Key Proteins in Blood Stem Cell Replication
A family of cancer-fighting molecules helps blood stem cells in mice decide when and how to divide, say researchers at the Stanford University School of medicine. Blocking the molecules’ function spurs the normally resting cells to begin proliferating strangely - making too much of one kind of cell and not enough of another. Many types of human blood cancers involve a similar disruption in the expression of that same family of molecules.
The blood stem cells’ misguided enthusiasm also inhibits their ability to successfully repopulate the immune system of a recipient animal after a bone marrow transplant - a common leukemia treatment.
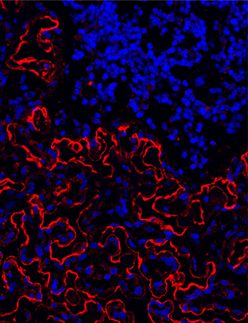
The discovery is the first to directly link the notorious members of the retinoblastoma family of proteins to the cellular production factories responsible for churning out all the blood and immune cells in the body. “This is an important step in understanding the initiation of human cancer at a cellular level,” said Patrick Viatour, PhD, a postdoctoral scholar who performed the research in the laboratory of Julien Sage, PhD.
Sage, assistant professor of pediatrics and of genetics, recently received a SEED grant from the California Institute of Regenerative Medicine to investigate how the retinoblastoma, or Rb, proteins affect human embryonic stem cells. Viatour is the first author of the research, which was published in the Oct. 9 issue of Cell Stem Cell.
“These studies, and additional experiments from our lab in other tissues and organs, indicate that Rb proteins play a critical role in suppressing tumors originating in adult stem cells populations,” said Sage, who is also a member of the Stanford Cancer Center.
The first retinoblastoma protein, pRb, was identified through studies of retinal cancer arising in children in whom the protein is missing or mutated. Since that time, Rb proteins have been shown to be involved in preventing many different types of human cancers. Further study showed that pRb stops a cell from dividing before it has appropriately duplicated and segregated its genetic material - coordinating the complex series of events like a traffic light at a busy intersection.
The protein doesn’t work alone, however. Two other family members, p107 and p130, also help carry out the important duties. Their ability to fill in for one another makes it difficult to parse out exactly what the proteins are doing at a molecular level. Unfortunately, laboratory animals missing just one or two family members die soon after birth.
Viatour and Sage devised a way to inhibit, or knock out, the function of all three proteins in adult mice. They genetically engineered animals in which the p107 gene is deleted and the pRb and p130 genes are flanked by pieces of DNA that are recognized and cleaved by a specialized protein called the Cre recombinase. When expressed in blood stem cells, the recombinase snips out the Rb and p130 genes, leaving these stem cells and their progeny - that is, the entire blood system - without any functional Rb family members.
The researchers found that blood, or hematopoetic, stem cells in the mice, which usually hang around quietly waiting to be called into action, began actively proliferating when Rb family members were missing. And while unmodified blood stem cells give rise to two main groups of cells - myeloid and lymphoid - the cells missing the Rb family strongly favored the myeloid lineage.
“The differentiation of these hematopoetic stem cells is clearly defective,” said Viatour, who also collaborated with bioinformatician and pediatrician Atul Butte, MD, PhD, and many other Stanford researchers on the work. Butte, an assistant professor of medicine and pediatrics, helped the researchers investigate the gene expression profiles of the blood stem cells. “We found that key myeloid genes were upregulated in the cells, and that lymphoid-associated genes were downregulated,” said Viatour. In contrast, the ability of the stem cells to make more of themselves seems unimpaired.
Finally, in an experiment mimicking human bone marrow transplantation, hematopoetic stem cells from the mice missing the Rb family members were no longer able to repopulate the immune systems of animals that had received a lethal dose of radiation.
“It’s been known that resting, or quiescent, stem cells are much more likely to be successful candidates for transplantation in both humans and mice than are actively dividing cells,” said Viatour. “We now have a good model for understanding why that is.”
Viatour and his collaborators plan to continue investigating how the Rb family members affect hematopoetic stem cells. One challenge will be to develop an organ-specific way to knock out Rb family function. They’d also like to find out why differentiated myeloid cells don’t also proliferate inappropriately, since they too are missing Rb family members.
Other Stanford co-authors include postdoctoral scholars Tim Somervaille, MD, PhD, and Shivkumar Venkatasubrahmanyam, PhD; and the director of Stanford’s Stem Cell Biology and Regenerative Medicine Institute, Irving Weissman, MD. Emmanuelle Passegue, PhD, was a postdoctoral scholar in Weissman’s lab when the work was conducted; she is now an assistant professor of medicine at UC-San Francisco.
The research was supported by the National Institutes of Health, the Lucile Packard Foundation for Children’s Health, the Damon Runyon Cancer Foundation, the California Institute for Regenerative Medicine, the Human Frontier Science Program, the European Molecular Biology Organization, the Fonds de la Recherche Scientifique, the Leon Fredericq Foundation and the Leukemia and Lymphoma Society.