Elan and Biogen Idec Initiate First Clinical Trial of TYSABRI in Oncology
Elan Corporation, plc and Biogen Idec announced the initiation of the first clinical trial of TYSABRI® (natalizumab) in oncology. The first dose of TYSABRI was administered in the trial. The objectives of this Phase I/II study are to evaluate the safety and potential anti-tumor activity of TYSABRI in patients with relapsed or refractory multiple myeloma. TYSABRI is a recombinant, humanized monoclonal antibody that targets the adhesion molecule VLA4 (also known as alpha-4 integrin) that is expressed on the surface of many types of immune cells. VLA4 is also found on the surface of multiple myeloma cells and may be involved in their survival.
This Phase I/II, open-label, two-arm study is designed to evaluate the safety and anti-tumor activity of TYSABRI in patients with relapsed or refractory multiple myeloma. In the Phase I portion of the trial, a standard dose-escalation design will be used to assess the safety and tolerability of TYSABRI in up to 12 patients. In the Phase II portion of the study, up to 30 patients will be randomized to the tolerated doses identified in Phase I of the study.
Treatment cycles will consist of intravenous infusions of TYSABRI once every 28 days for 6 months. After 6 months, if the patient has achieved a partial or a complete response, he or she may continue to receive TYSABRI once every 28 days until progression of disease occurs.
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
New Phase II data show Novartis' ACZ885 gave better pain relief and flare prevention for patients with chronic gout
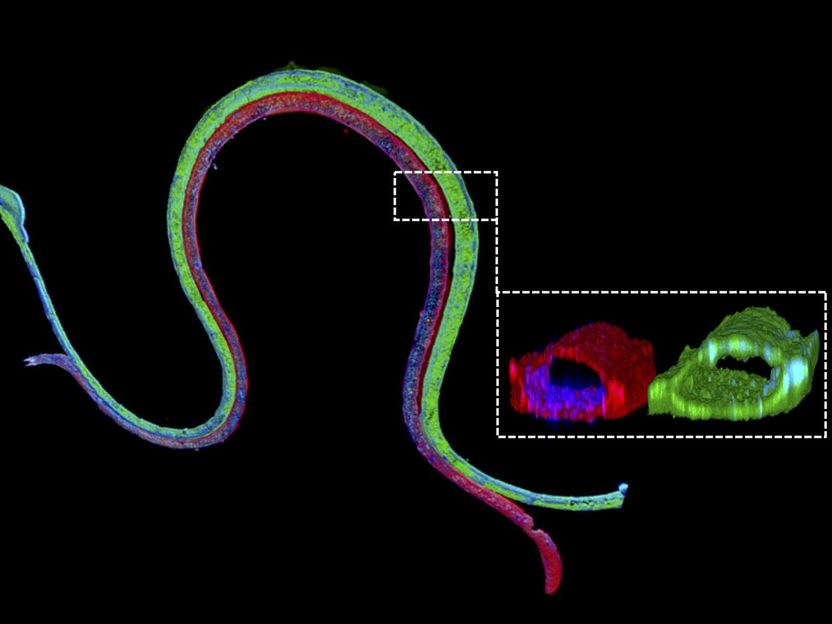
Renal reabsorption in living devices - 3D bioprinted, vascularized proximal tubules mimic the human kidney's reabsorption functions
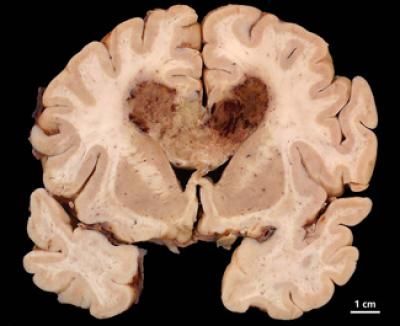
Brain cancer cells hide while drugs seek - Tumor cells temporarily lose mutation to evade drugs targeting mutation
FORMAC strengthens management with appointment of CSO and COO
Experimental drug shows promise against brain, prostate cancers

Right under your nose: A more convenient way to diagnose Alzheimer's disease - Certain proteins in nasal discharge can indicate the onset and progression of Alzheimer's, providing an avenue for early detection
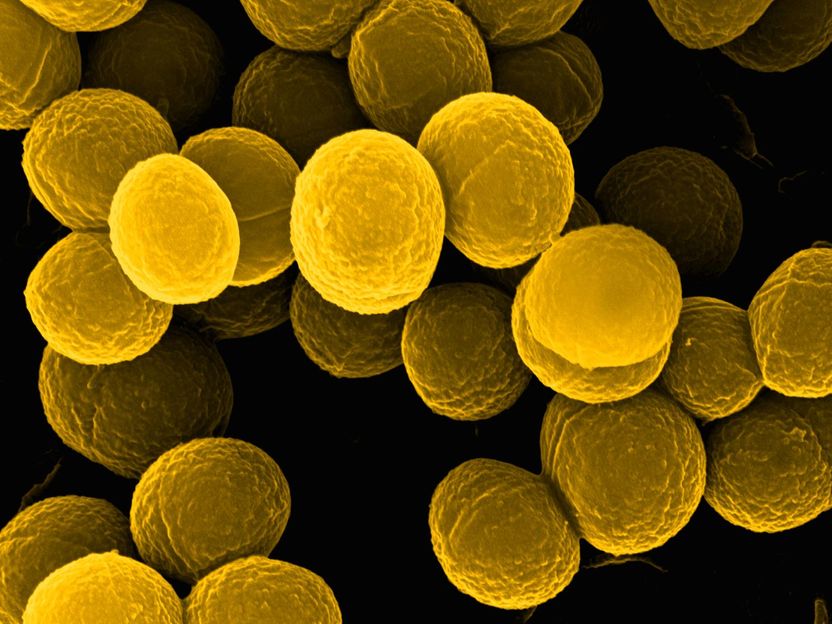
A bright spot for microbiological diagnostics - Researchers develop molecular probes to detect pathogens in clinical samples

New findings on the enzyme that breaks down PET plastic - Scientists increase efficiency: Start-up company in preparation
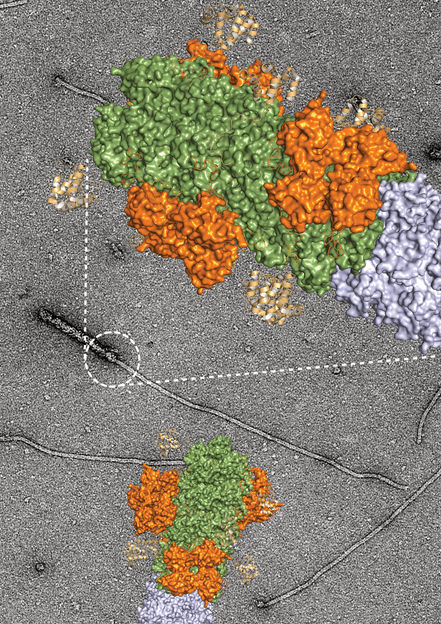
"Growing end" of inflammation discovered - Stopping chronic inflammatory diseases
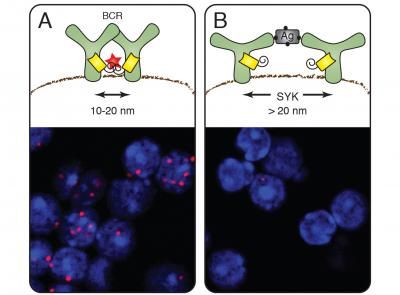
Nanoscale ruler reveals organization of the cell membrane - Freiburg biologists measure distances and the arrangement of membrane molecules in nanometer range

























































