NicOx announces U.S. phase 2a results for PF-03187207 and gives an update on continuing NO-prostaglandin program
NicOx S.A. announced the results of a U.S. phase 2 study, conducted by its partner Pfizer Inc, which compared the safety and efficacy of various doses of PF-03187207 to Xalatan® (latanoprost) 0.005% in patients with primary open-angle glaucoma and ocular hypertension. The higher doses of PF-03187207 demonstrated a clinically significant reduction in diurnal intraocular pressure (IOP) from baseline and the highest dose showed consistently more IOP lowering than Xalatan® 0.005%, at all study visits and at all individual time points, suggesting a beneficial effect of nitric oxide donation. PF-03187207 is a nitric oxide-donating prostaglandin analog, which is covered by the companies' August 2004 agreement.
On the primary endpoint at 28 days, PF-03187207 showed a 12% improvement over Xalatan® 0.005% which did not reach statistical significance. However, a statistically significant advantage over Xalatan® 0.005% was observed on a number of secondary endpoints (p<0.05).
Pfizer has taken the decision not to launch a global phase 3 development program for PF-03187207. Nevertheless, Pfizer has reaffirmed its commitment to the ongoing joint research program with NicOx, which aims to identify the most active nitric oxide-donating prostaglandin analogs for development on a global basis.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Cultivation of GM crops increases worldwide, but Europe is missing out
Amyotrophic_lateral_sclerosis
Ferriman-Gallwey_score
GE Healthcare completes acquisition of Whatman plc
Osmotic_demyelination_syndrome
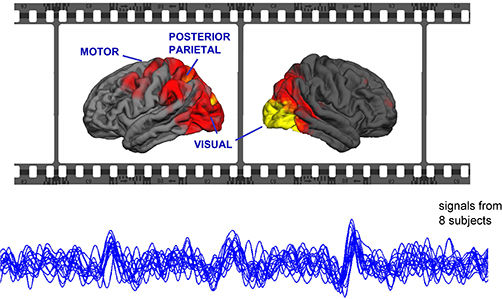
Movies synchronize brains - When we watch a movie, our brains react to it immediately in a way similar to other people's brains.
Innovative genetic and cellular techniques help identify multiple disease targets
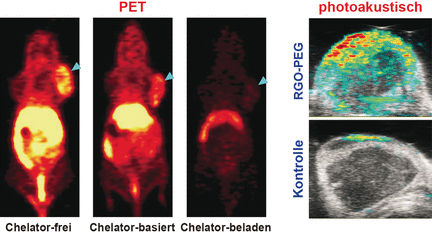
Direct radiolabeling of nanomaterials
Research will speed tracing bacteria behind salmonella outbreaks