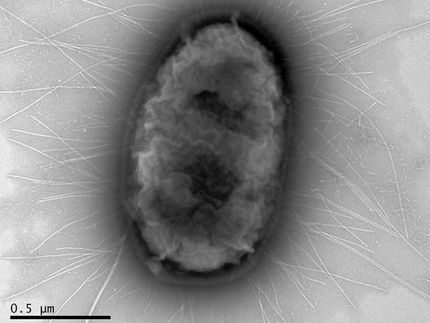
Transplantation Safety: New Virus Detected Using Genome Sequencer Technology
454 Life Sciences, a part of Roche Applied Science, announced that researchers at Columbia University and Victorian Infectious Disease Reference Laboratory have discovered a new virus that was responsible for the deaths of three transplant recipients. The research explains how the previously unknown virus, which is related to lymphocytic choreomeningitis virus, was published New England Journal of medicine. The study entitled, "Discovery and implication of a novel arenavirus in a cluster of fatal transplant associated disease," describes how identification of this virus might enable improvements in screening that will enhance the safety of transplantation.
Following the deaths of all three recipients of organs from a single donor, their illnesses were investigated by unbiased high throughput 454 Sequencing to reveal the presence of a previously undiscovered old world arenavirus. The virus, similar to lymphocytic choreomeningitis virus and Kodoko virus, was subsequently confirmed in multiple clinical samples by PCR, viral culture and immunohistochemistry. Results of the study implicate this new arenavirus as a human pathogen and support the use of high throughput sequencing in pathogen discovery.
"Within a few days we had clues to the identity of the virus responsible for transplant deaths" said Ian Lipkin, MD, lead author of the study and John Snow Professor of Epidemiology and Professor of Neurology and Pathology at Columbia University and director of the Center for Infection and Immunity at the Mailman School of Public Health. "We made this investigation highest priority. Our success reflected the tireless efforts of an extraordinary team of investigators representing academia, public health and industry, who brought all of their expertise and technology to bear on an important clinical problem."
Lymphocytic choreomeningits virus has been implicated in other transplant outbreaks; however, the newly discovered virus is sufficiently different that it could not be detected using existing screening and traditional sequencing methods all requiring prior knowledge of exact sequence of the suspected pathogen.
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Affymetrix Selected to Genotype More Than 9,000 Framingham Heart Study Samples - SHARE Project to Help Identify Genetic Variants Associated With Heart, Lung, Blood and Sleep Disorders

New app calculates corona infection risk in rooms - Size of aerosol droplets that virus carriers release strongly influences infectivity
Northern_Norway_Pharmaceutical_Trust

Cell culture: The opportunities and the challenges - The promise of cell culture is accelerating demand, and emphasising the challenges, of this process
Crucell Teams up with ACE BioSciences and Harvard to Accelerate Bacterial Antibody Program
St_John_Ambulance_in_England_and_Wales
Harold_Ridley_(ophthalmologist)
Henry_Suter