Advanced Cell Technology Announces Creation of Human Embryonic Stem Cell Lines Without the Destruction of Embryos
Breakthrough Approach Improves Efficiency to Levels Reported in the Conventional Stem Cell Derivation Techniques
Advanced Cell Technology, Inc. together with colleagues announced the development of five human embryonic stem cell (hESC) lines without the destruction of embryos. These new results have the potential to end the ethical debate surrounding the use of embryos to derive stem cells. In fact, the NIH report to the President refers to this technology as one of the viable alternatives to the destruction of embryos.
The new method will be published in the journal Cell Stem Cells, published by Cell Press. The peer-reviewed technique was initially carried out by ACT scientists under the direction of Robert Lanza, M.D., and then independently replicated by scientists on the West Coast. Single cells were removed from the embryos using a technique similar to preimplantation genetic diagnosis (PGD). The biopsied embryos continued to develop normally and were then frozen. The cells that were removed were cultured utilizing a proprietary methodology that recreates the optimal developmental environment, which substantially improved the efficiency of deriving stem cells to rates comparable to using the traditional approach of deriving stem cells from the inner cell mass of a whole blastocyst stage embryo. The stem cells were genetically normal and differentiated into cell types of all three germ layers of the body, including blood cells, neurons, heart cells, cartilage, and other cell types of potentially therapeutic significance.
"This is a working technology that exists here and now," said Robert Lanza, M.D., Chief Scientific Officer at Advanced Cell Technology and senior author of the paper. "It could be used to increase the number of stem cell lines available to federal researchers immediately. We could send these cells out to researchers tomorrow. If the White House approves this new methodology, researchers could effectively double or triple the number of stem cell lines available within a few months. Too many needless deaths continue to occur while this research is being held up. I hope the President will act now and approve these stem cell lines quickly."
The paper also addresses several other important issues. First, the stem cells were derived without culturing multiple cells from each embryo together, and at efficiency levels similar to that reported for conventional stem cell derivation techniques using blastocysts. Second, it addresses ethical objections that the derivation system required co-culture with hESCs from other embryos that were destroyed. The current study demonstrates that hESC co-culture is not an essential part of the derivation procedure. The stem cell lines generated in the present study appear to have the same characteristics as other hESC lines, including expression of the same markers of pluripotency, self-renewing capacity, genetic stability, and ability to differentiate into derivatives of all three germ layers of the body.
"We are excited that our new method for generating human embryonic stem cell lines without the destruction of embryos has been accepted for inclusion by such a prestigious publication," said William M. Caldwell IV, Chairman and CEO of Advanced Cell Technology. "This new approach addresses the President Bush's ethical concerns. We are hopeful that the NIH will consider this new approach for federal funding. We believe that such consideration reflects the desire of the American people to bring therapies derived from stem cell research to patients with few or no alternatives."
Other contributors to the study and publication include Young Chung and Irina Klimanskaya, Sandy Becker, Tong Li, Marc Maserati, and Shi-Jiang Lu of Advanced Cell Technology; Tamara Zdravkovic, Olga Genbacev, and Susan Fisher of the University of California, San Francisco; and Dusko Ilic and Ana Krtolica of StemLifeLine.
Topics
Organizations
Related link
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
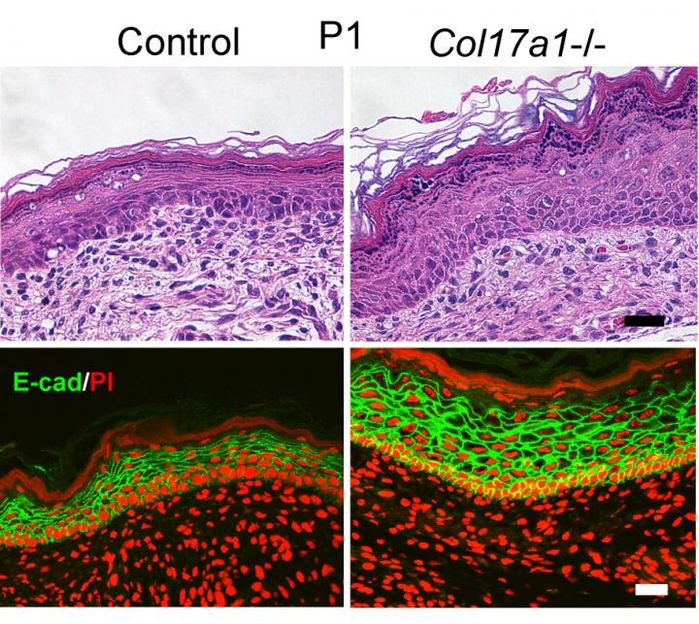
Collagen controlling the thickness and juvenile state of skin
Journal_of_Bone_&_Joint_Surgery
Category:Marine_biologists

Ionic and covalent drug delivery
Bothriopsis
Evotec Completes Divesture of Chemical Development