Santhera Reports Encouraging, First Data from Phase IIa Clinical Trial with SNT-MC17 in Duchenne Muscular Dystrophy
Santhera Pharmaceuticals announced positive, first results from a 12 month Phase IIa clinical trial with SNT-MC17 (INN: idebenone) in Duchenne muscular dystrophy (DMD) as measured by cardiac and respiratory parameters. Santhera therefore is committed to further clinical development of SNT-MC17 in DMD.
This exploratory Phase IIa trial was a 12-month double-blind, randomized, placebo-controlled study conducted at the University of Leuven, Belgium. In total 21 DMD patients between the age of 8 and 16 years were enrolled to assess the efficacy and tolerability of one dose level of SNT-MC17 (450 mg/day) compared to placebo. Thirteen patients were receiving SNT-MC17 while 8 patients were randomized to the placebo group. There were no drop-outs in the study and the compliance was very good. Importantly, there was no difference in the safety and tolerability of SNT-MC17 compared to placebo underlining again the excellent safety profile of SNT-MC17 also in this pediatric population.
The primary objective was to assess whether SNT-MC17 improves or slows the decline in cardiac function in DMD patients, applying a comprehensive echocardiographic approach that included cardiac tissue Doppler and strain rate imaging technology. The primary endpoint was an assessment of the change in contractility of the region of the heart muscle that is affected early and most severely in DMD patients, measured by the peak systolic radial strain of the left ventricular inferolateral wall. After treatment for twelve months with SNT-MC17, DMD patients showed a trend to improve on this functional cardiac parameter compared to placebo (P=0.098).
In addition to these data, patients on SNT-MC17 improved also on certain respiratory parameters. Most striking and statistically significant was the improvement of DMD patients' lung function measured by peak flow (P=0.042). Patients treated with SNT-MC17 ameliorated on this parameter, while patients on placebo deteriorated over the study period.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Cancer Target Collaboration Agreement Signed Between MedImmune and Georgetown University
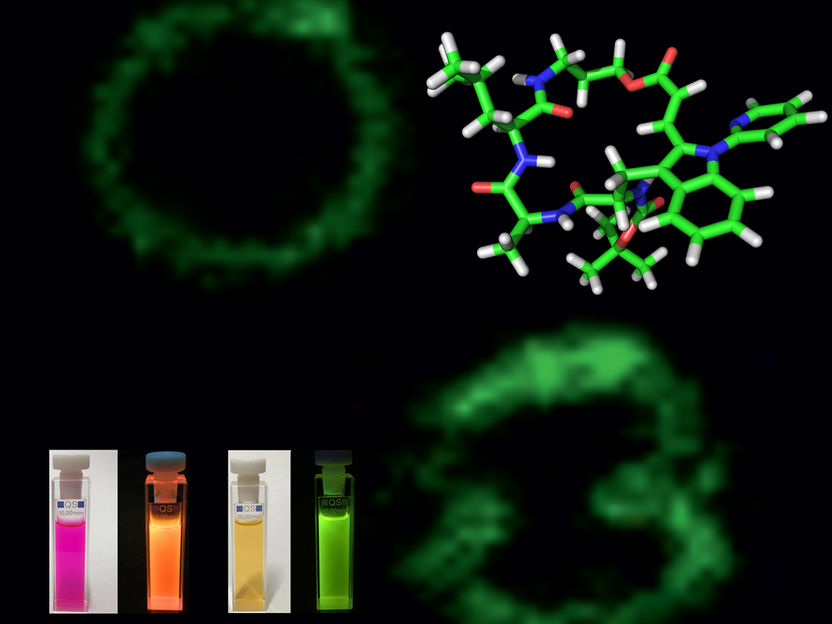
Clever biomolecular labelling enables identification of immune cells - New strategy for labelling peptides
Laminopathies: Key components in the disease mechanism identified
CLC bio establishes independent non-profit foundation to give back to the genomics community