OctoPlus announces positive Phase IIa efficacy and tolerability results for Locteron in hepatitis C
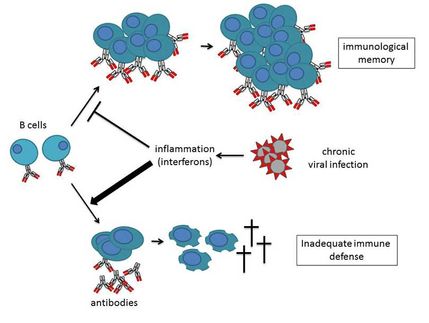
OctoPlus N.V. announced the successful completion of the Phase IIa clinical study with its lead product Locteron(TM), a controlled release interferon alfa product for the treatment of chronic hepatitis C (HCV).
According to the company, results from this study confirm Locteron's potential to substantially improve patient care in HCV. Safety data from the study show a substantially improved tolerability profile for Locteron compared to other interferon products on the market or in development. Efficacy data from the study indicate Locteron's strong antiviral effect, with 100% of the patients achieving early virologic response in the two highest dose groups. In addition, Locteron is a more convenient therapy than existing treatments due to its controlled-release profile, allowing for once every two weeks drug administration versus the current once a week regimen. OctoPlus is co-developing Locteron with its partner Biolex Therapeutics.
Locteron is designed to be a best-in-class therapeutic for patients with chronic hepatitis C, with the potential to induce less side effects, improve patient compliance and provide a more convenient once every two week dosing schedule compared with current therapies. Locteron combines OctoPlus' proprietary PolyActive(TM) drug delivery technology with BLX-883, a recombinant interferon alfa produced by OctoPlus' co-development partner Biolex Therapeutics in its patented LEX System(SM). Locteron is produced in OctoPlus' cGMP manufacturing facilities in Leiden, the Netherlands.
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Cytori Reports Promising Results in Cell-Enriched Breast Reconstruction Trial
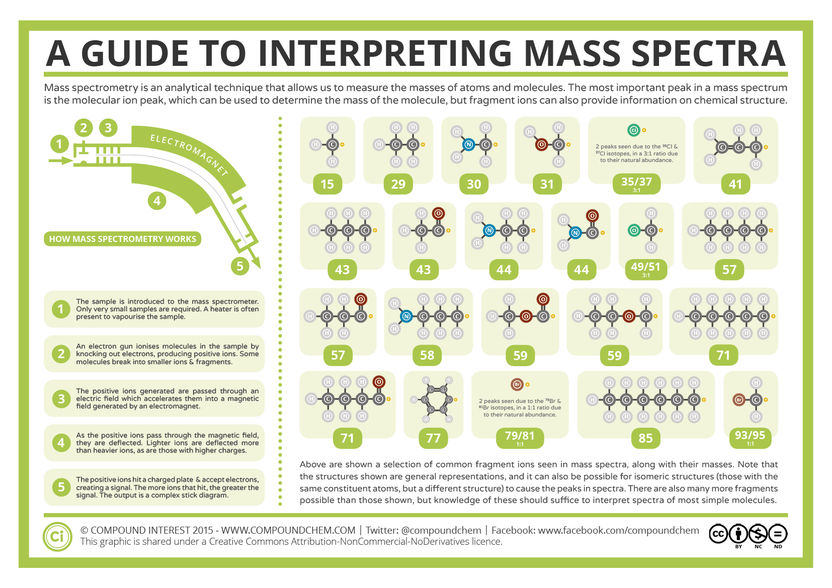
Mass Spectrometry and Interpreting Mass Spectra
Category:Medical_conditions_related_to_obesity
ClinTec’s CEO, Dr Rabinder Buttar wins the Institute of Directors, Director of the Year Award for Glasgow & West of Scotland
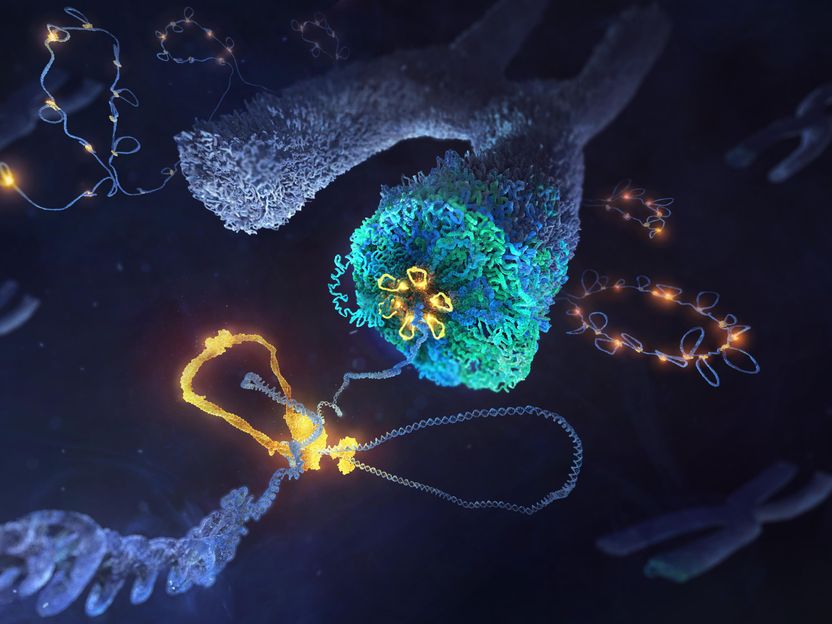
Shaping up the Genome for Cell Division - Researchers have uncovered the inner workings of the molecular machinery that shapes chromosomes during cell division
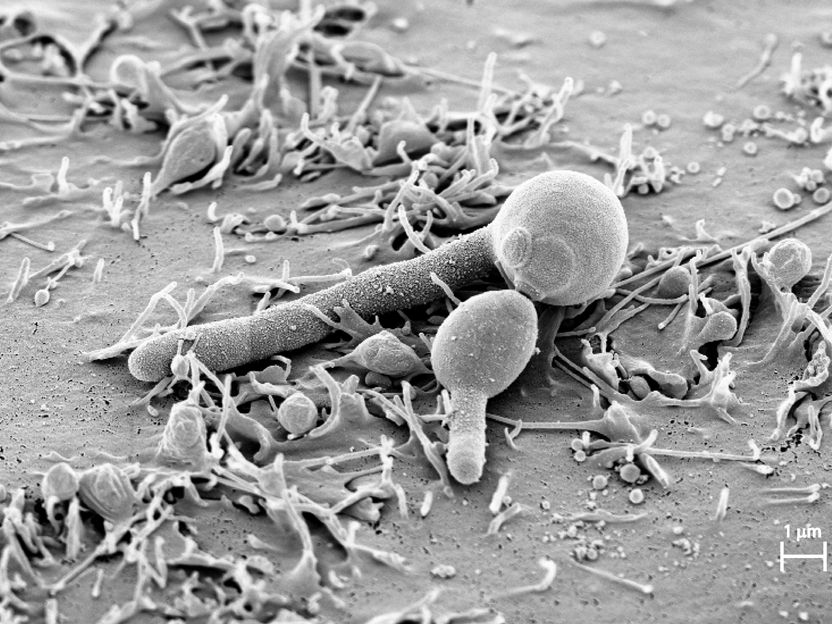
Candidalysin – the first toxin of Candida albicans - Team of German and British scientists discovers tissue-damaging fungal toxin

Wacker Chemie AG: Chemical and biotechnology divisions performed in a weak market environment in 2024 - Preliminary figures