Potent Peptides Inhibit HIV Entry Into Cells
Protein analysis at Brookhaven's light source helps researchers in design of new AIDS drugs
Based in part on protein structures determined at the National Synchrotron Light Source (NSLS) at the U.S. Department of Energy's Brookhaven National Laboratory, scientists at the University of Utah have developed new peptides that appear to be significantly more effective at blocking HIV's entry into cells than other drugs in their class. In a paper in Proceedings of the National Academy of Sciences, the researchers say these peptides are sufficiently potent to begin pre-clinical studies as a new class of agents for the prevention and treatment of HIV/AIDS.
Brookhaven National Laboratory
"Our 'D-peptides' offer several potential therapeutic advantages over existing peptide entry inhibitors, which are costly, require high dose injections, and suffer from the emergence of drug-resistance," said University of Utah biochemist Michael S. Kay, lead author on the paper. "In contrast, our D-peptides resist degradation, so they have the potential to be administered by mouth and last longer in the bloodstream. Since these inhibitors have a unique inhibitory mechanism, they should work well in combination with existing HIV inhibitors."
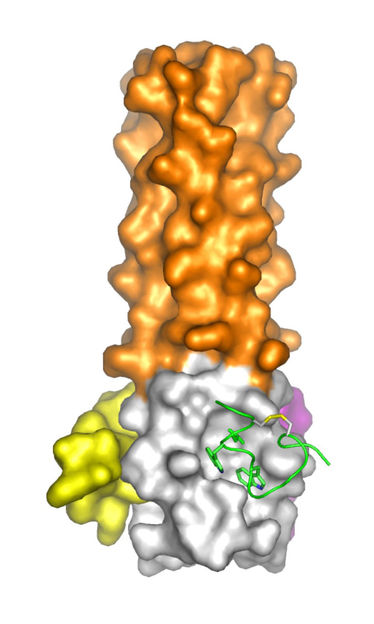
The researchers were particularly interested in developing drugs to bind to an essential "pocket" structure found in all HIV strains that was previously identified as a promising drug target using structures determined at Brookhaven's NSLS. Numerous previous attempts to target this pocket failed to produce potent and non-toxic pocket-specific entry inhibitors. In the current work, the researchers used a high-throughput technique to screen a "library" containing hundreds of millions of peptides to identify the rare peptides that would bind to the pocket structure and inhibit HIV entry.
After identifying the most promising candidate peptides, the researchers analyzed the structure of these peptides bound to the target protein using x-ray crystallography at the NSLS. In this technique, researchers analyze how an extremely bright beam of x-rays, available only at synchrotron sources, bounces off and is refracted by the sample to determine the positions of individual atoms. "These structures reveal details of how the peptides bind and guide the development of future inhibitors," said paper co-author Annie Heroux, a biologist and crystallography specialist at Brookhaven Lab.
This structure-assisted design led to the discovery of D-peptides with up to a 40,000-fold improved antiviral potency over previously reported D-peptides. The structures also suggest ways to engineer the peptides to reduce the chance of drug resistance
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Protein analytics
Protein analytics provides a deep insight into these complex macromolecules, their structure, function and interactions. It is essential for discovering and developing biopharmaceuticals, understanding disease mechanisms, and identifying therapeutic targets. Techniques such as mass spectrometry, Western blot and immunoassays allow researchers to characterize proteins at the molecular level, determine their concentration and identify possible modifications.

Topic world Protein analytics
Protein analytics provides a deep insight into these complex macromolecules, their structure, function and interactions. It is essential for discovering and developing biopharmaceuticals, understanding disease mechanisms, and identifying therapeutic targets. Techniques such as mass spectrometry, Western blot and immunoassays allow researchers to characterize proteins at the molecular level, determine their concentration and identify possible modifications.























































