Acambis' universal influenza vaccine enters Phase 1 trial
Acambis plc announces that it has initiated a Phase 1 clinical trial of its universal influenza vaccine candidate, ACAM-FLU-A.
The vaccine candidate is designed for targeting all 'A' strains of the influenza virus. As such, it has the potential to be both a universal pandemic influenza vaccine and part of a universal seasonal vaccine. Historically, influenza pandemics have been caused by 'A' strains of the virus while seasonal vaccines target both 'A' and 'B' strains of the virus. The Phase 1 trial is: - A randomised, double-blind, placebo-controlled, multi-centre trial in the US;
- Investigating ACAM-FLU-A's safety, tolerability and ability to generate an immune response;
- Being conducted in up to 80 healthy subjects aged between 18 and 40 years; and
- Assessing two adjuvants or "immune stimulants" to determine whether they improve the effect of the vaccine on the immune system.
ACAM-FLU-A is the first vaccine candidate being developed under Acambis' influenza programme. It is a recombinant vaccine that uses a hepatitis B core protein to deliver M2e, the extracellular domain of the ion channel protein M2. Being highly conserved, M2e is intended to elicit immune responses to all influenza 'A' strains. Acambis is using multiple approaches to develop innovative influenza vaccines. In addition to ACAMFLU-A, its programme includes activities to identify an equivalent conserved region within the influenza virus 'B' strains in order to generate a universal vaccine candidate protective against all the strains that cause the high annual morbidity and mortality in humans. Acambis is also exploring recombinant neuraminidase- and haemagglutinin-based vaccines and their delivery using proprietary platform technologies to develop vaccine candidates superior to existing seasonal influenza vaccines.
Acambis' vaccine candidate was developed using technology licensed from VIB that was invented by Walter Fiers, Emeritus Professor of the VIB Department for Molecular Biomedical Research at the University of Ghent, Belgium.
As part of Acambis' plans to test the effect of various adjuvants on the safety and immunogenicity of ACAM-FLU-A, two adjuvants are being tested in this Phase 1 trial: aluminium hydroxide, which is widely used in licensed vaccines; and QS-21 Stimulon® adjuvant, which is an investigational adjuvant provided under a licence and option agreement with Antigenics Inc.
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Fulvio Mavili is appointed as Généthon's new Scientific Director
MWG Biotech AG successful in important patent dispute for thermocycler apparatuses.

CEFLA S.C. - Imola, Italy
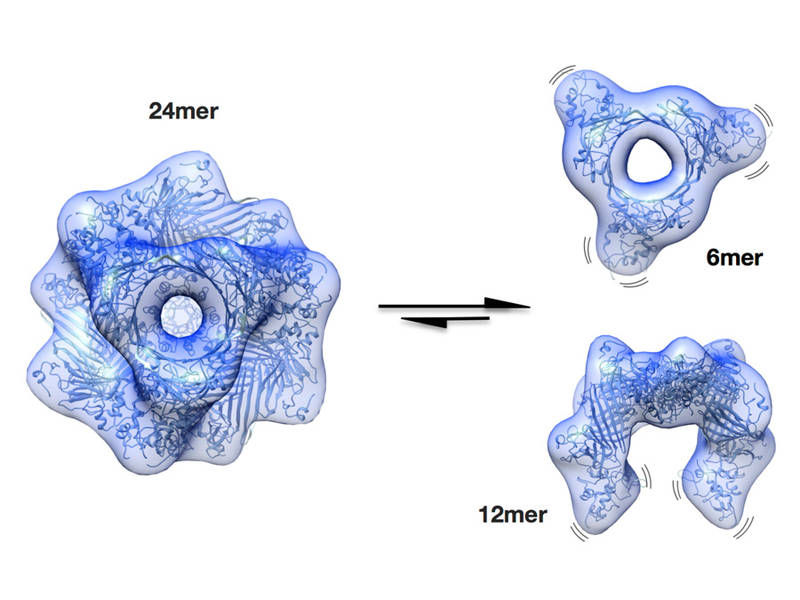
A protein safeguards against cataracts - Activation mechanism of a protective protein in the ocular lens resolved

Ideaya Biosciences - South San Francisco, USA