EU Regulation Urgently Needed
European Patients are Waiting for the Next Generation of Medical Treatments
Representatives from European patients' organisations, academia, science and industry urged Europe's decision-makers to approve the common framework for "Advanced Therapies" - treatments made from cells, genes or tissue engineering - last evening at a dinner debate organised by EPPOSI, hosted by Mrs. Avril Doyle, Member of the European Parliament.
More than 9 MEPs and/or their Political Assistants gathered to listen to points of view from across the stakeholder spectrum - including patients' representatives, scientists developing treatments for rare genetic diseases and representatives of the Life Sciences sector - to understand why the Regulation was important to them. And the overwhelming message was: this Regulation is needed and it is needed now.
The draft Regulation is currently under discussion at the European Parliament, which is expected to vote on 13th March 2007.
"Advanced Therapies have the potential to revolutionise the way that patients are treated and, in many cases, offer treatments where none existed previously," explained Mrs. Doyle. "This Regulation is the key to getting those products out of the laboratories and making sure that they are safely available to the patients," she confirmed.
Currently, each individual Member State of the EU regulates these products differently. In some countries, they are not regulated at all, meaning that patients cannot be sure of the safety of products that they might be receiving. Therefore, patients urged that all products should be subject to the evaluation. "It is unacceptable that some products are left in the unregulated grey zone," explained Alastair Kent, president of EGAN, the European Genetic Alliances Network. "Delaying the approval of the Regulation, or excluding some products from this Regulation will prevent some patients having access to them. But worse, those that do get them will have no guarantee of their safety."
And for the Life Sciences sector, the patchwork of rules and regulations means that each product must be registered country by country. Evidence must be gathered and formatted according to each individual system, meaning that patients in one country will receive the treatment long before others as the registration must be done step by step.
The new EU Regulation would solve all of this by putting in place a single EU evaluation - similar to that currently required for normal medicines - which would allow patients all over the EU assurances of safe, efficacious and high-quality products all at the same time.
The draft, endorsed by the European Parliament's own Environment Committee which has primary responsibility for the draft legislation, calls for all products to be evaluated to the same standards, nevertheless leaves it up to individual Member States to accept the product in its market or not. "This is the same approach as that taken with contraceptive products, where views differ from one country to another," continued Alastair Kent.
Other news from the department politics & laws
These products might interest you

Systec H-Series by Systec
Safe, reproducible and validatable sterilization of liquids, solids and waste
Autoclaves with 65-1580 liters usable space, flexibly expandable for various applications

Whatman™ folded filter papers by Cytiva
Whatman folded filter papers
Convenient folded formats speed up your sample preparation

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Got good fat?
Ultrafast lasers protect adenine - Ultrafast lasers can protect DNA building block from light-induced dissociation
How can we fight blood cancer more effectively? - Several hundred different treatment combinations to be tested simultaneously outside the body

B Medical Systems S.à r.l. - Hosingen, Luxembourg
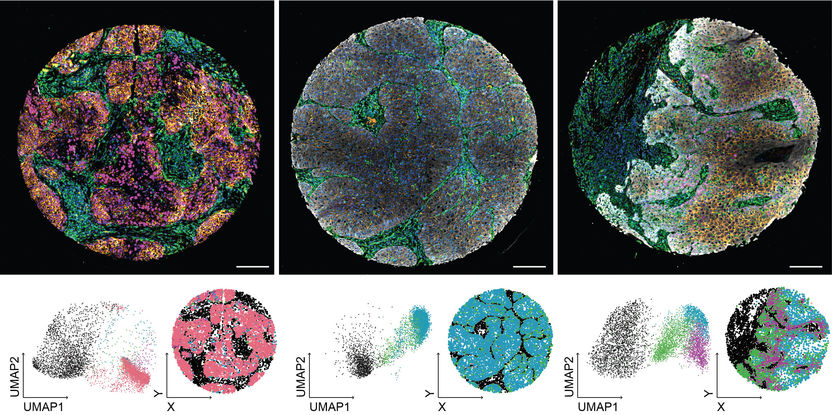
New imaging analysis method revolutionizes the diagnosis of head and neck tumors
Scientific Paradox: Phosphate Increases The Concentration of Sodium (!) in The Blood
BASF Plant Science takes Amflora case to EU Court - BASF filed action against the EU Commission for failure to act
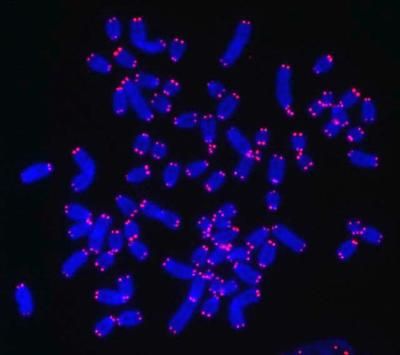
A double ring ceremony prepares telomerase RNA to wed its protein partner

Using light to produce medication and plastics more efficiently - How the energy efficiency of photochemical reactions can be increased tenfold
AMT's Lead Product Poised to Address Major Liver Disease - European Patent Office Grants Patent for Treatment of Non-alcoholic Steatotic Hepatitis