Basilea Announces Start of Phase III Program for Isavuconazole
Basilea Pharmaceutica Ltd. announced the opening of two primary-treatment phase III clinical trials for isavuconazole (BAL8557) in patients with invasive systemic fungal infections caused by yeasts and molds. The global trial program for isavuconazole (BAL8557) focuses on patients with invasive candidiasis including candidemia and patients with proven or probable aspergillosis. Isavuconazole was granted fast-track designation by the U.S. Food and Drug Administration (FDA).
According to the company, Isavuconazole has a potent and broad spectrum of activity against both yeasts and molds. This new triazole is developed as a water-soluble pro-drug to allow safe and simple intravenous administration without contraindication in renally impaired patients. In addition, taken as convenient once daily or once weekly capsules, the prodrug results in rapid and almost complete absorption and distribution to infected tissues.
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Molecular farming fits need for fully functional protein therapeutics and low-cost Vaccines, says Frost & Sullivan
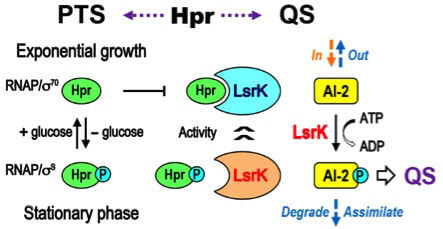
Link between bacteria metabolism and communication could pave way for new antivirulence drugs

Oncgnostics cooperates with the Medical University of Graz - Study on Head-and-Neck tumors aims to demonstrate that a diagnostic method can reliably and early detect malignant tumors using non-invasive saliva samples
Portopulmonary_hypertension
Zygosity
The Number of Biotechnology Companies has Only Decreased by 10% Despite the Crisis
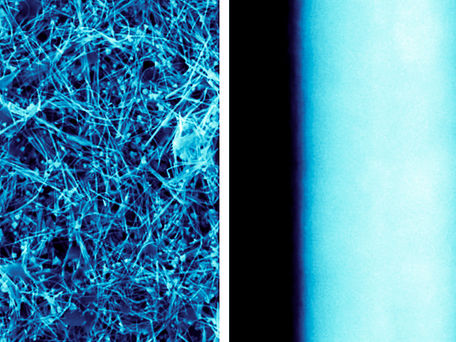
Scientists use copper nanowires to combat the spread of diseases
Univar Czech s.r.o. acquires enzymes distribution business of Ekozym
Scientists identify gene for deadly inherited lung disease
Meiosis
US Government Exercises Next Part of Bavarian Nordic's Freeze-Dried IMVAMUNE Contract






















































