Scientists Convert Modern Enzyme into its Hypothesized Ancestor
Single amino acid substitution supports theory of common origin some 2.5 billion years ago
By making a single substitution in the amino acid sequence of a modern enzyme, scientists have changed its function into that of a theoretical distant ancestor, providing the first experimental evidence for the common origin of the two distinct enzyme types. The research, conducted by a team that includes scientists from the U.S. Department of Energy's Brookhaven National Laboratory and the Karolinska Institute in Stockholm, Sweden, will be published in 'Proceedings of the National Academy of Sciences'.

"It's as if we turned back the clock nearly 2.5 billion years, to the time when oxygen first appeared in Earth's atmosphere, to get a snapshot of how enzymes evolved to deal with reactive oxygen species," said Brookhaven biochemist John Shanklin, lead author on the paper. After the first photosynthetic organisms appeared on Earth some 2.5 billion years ago, pumping oxygen into the atmosphere, organisms with enzymes capable of deactivating reactive oxygen species had an increased chance of survival.
Scientists have theorized that the first oxygen-detoxifying enzymes were simple oxidases, which combine reactive forms of oxygen, such as peroxide, with hydrogen ions (protons) and electrons to yield water (H2O). While these enzymes have little in common with more modern biosynthetic enzymes that mediate oxygen chemistry, they share certain structural and sequence characteristics around their active sites - namely, a pair of iron atoms for binding oxygen within a similar four-helix bundle. These similarities suggested the possibility of a common origin, but experimental evidence was lacking - until now.
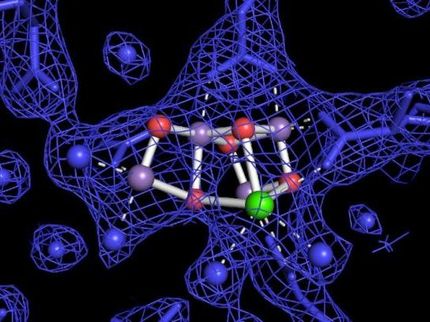
The Brookhaven/Karolinska team had previously performed a structural comparison of the active site of a modern desaturase enzyme (which uses activated oxygen to remove two hydrogens from fatty acids) with that of a simple peroxidase. They used a stand-in for oxygen binding in the active site (because oxygen itself does not stay bound long enough for studies) and produced molecular-level crystal structures using high intensity beams of x-rays at the National Synchrotron Light Source at Brookhaven Lab and the MAX Lab at the University of Lund Synchrotron in Sweden.
These crystal structures revealed remarkable similarities, with the single major difference being a change in one amino acid residue adjacent to the oxygen-binding site: The oxidase had an acidic residue capable of donating protons to the oxygen to form water while the desaturase did not.
Based on this difference, the scientists hypothesized that if they engineered a "desaturase" with an acidic amino acid residue in place of the non-reactive one, they would convert the desaturase to an oxidase. Using the tools of molecular biology, this is exactly what they did. "Substituting aspartic acid at this site on the desaturase made a huge change," Shanklin said.
The new enzyme's desaturase activity decreased 2000-fold while its oxidase activity increased 31-fold compared with the original desaturase. New crystal structures, derived at the European Synchrotron Radiation Facility in France, revealed that the substitution placed the acid group into the ideal position for donating protons to the oxygen.
"Usually, when enzymes evolve from a common ancestor, there are many amino acids that change to change the function," Shanklin said. "So it is remarkable that changing the identity of a single amino acid in an enzyme of 400 amino acids can make such a dramatic switch in the chemical reaction it performs. This finding, that such a simple change can dramatically alter function, provides experimental support for the hypothesis that these two enzyme groups share a common origin."
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.