Medivir spins out the antiviral compound MIV-606 to Epiphany
Medivir AB and Epiphany Biosciences announced the signing of a license agreement on MIV-606 (valomaciclovir), Medivir's phase II compound with potent activity against varicella zoster virus (VZV) and other viruses.
Under the terms of the agreement Medivir will receive equity in Epiphany, milestone payments of maximally USD 24.5m and royalty from world wide sales except the Nordic countries where Medivir has retained the marketing rights for all disease indications. Epiphany will be responsible for the further clinical development of MIV-606.
Phase IIa studies have shown MIV-606 to be efficacious and safe in patients with shingles, caused by the varicella zoster virus (VZV). According to Medivir, the market for zoster drugs is going to increase with the aging population. MIV-606 is also a potent inhibitor of other herpesviruses increasingly implicated in various diseases such as mononucleosis, chronic fatigue syndrome, multiple sclerosis and the development of HIV/AIDS.
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
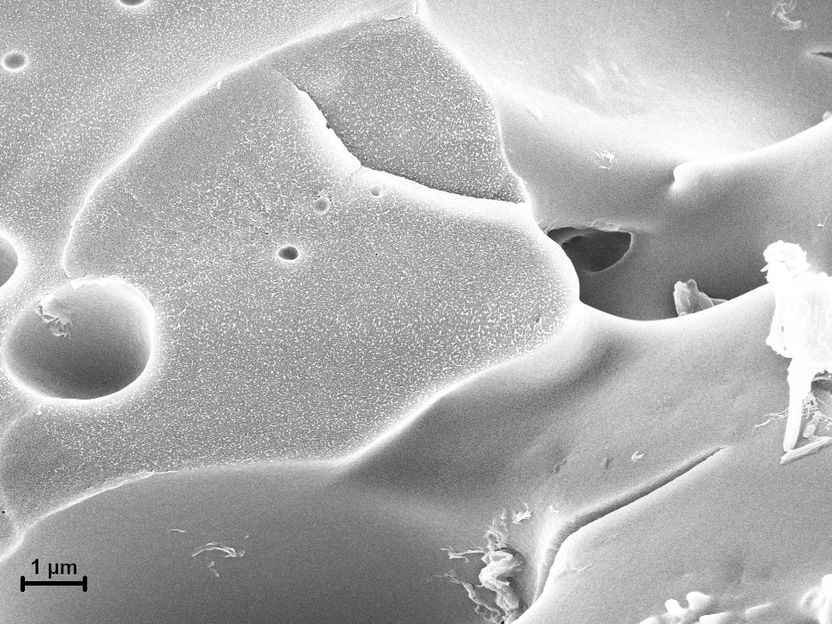
Self-healing plastic becomes biodegradable - Greater environmental compatibility thanks to new basic building block that can be produced with the help of microorganisms and is completely biodegradable























































