Campus Vienna Biocenter: Nanostructures of the Infective Apparatus of Salmonella
In Salmonella, structural changes to the molecular infection apparatus also signal an end to its further assembly. The mechanistic details of this sophisticated feedback system, which takes place at molecular level, have now been published. Better understanding of how this pathogen¹s needle-like secretion injectisome is formed will offer new approaches to preventing the infection process in future. The results obtained by the team of Thomas Marlovits, joint IMP-IMBA Group Leader and head of the new "Spot of Excellence" at the Campus Vienna Biocenter, have now been explained by using modern techniques of three-dimensional cryo electron microscopy.
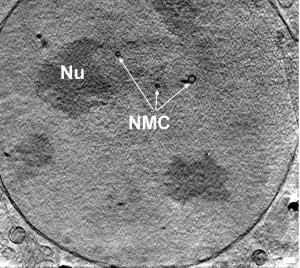
Salmonella cause typhoid fever and food poisoning. One of the key structural features of the infection process for this bacterium is the "type III secretion system" (TTSS). This enables it to secrete bacterial proteins into the host cell. The central component of this apparatus has a structure akin to that of a hollow needle, whose length is crucial for the success of the infection process.
Dr. Thomas C. Marlovits, scientific head of the new "Vienna Spot of Excellence", together with Prof. Jorge E. Galan (Yale University, USA) and other colleagues from the USA, has now explained how the exact length of the needle is determined during the assembly of this biological nano-machine. Says Dr. Marlovits: "A fine example of molecular multi-tasking, the TTSS is not only responsible for transporting bacterial proteins into the host cell, but also for its own assembly from some 200 individual structural proteins. The length of the needle structure is controlled by a sophisticated mechanism. The core of this mechanism is the change in the specificity of the TTSS for different proteins. Although the TTSS still has a high specificity for its own structural proteins during the initial phase of the assembly process, this specificity changes later to handle the proteins that are important for the actual infection process. A change in the structure of the TTSS is crucial for this transformation."
In actual fact, the TTSS comprises four important components: a base anchored into the bacterial membrane with a socket-like structure, plus an overlying inner ring structure on which the needle is built. Marlovits has now succeeded in demonstrating that the ring structure firmly binds the needle with the socket-like structure and the base. This bond also effects a structural change to the base, which impacts on its ability to bind to proteins from the cytoplasmic side of the cell. In this situation, the structural change acts as a signal to indicate that the needle is finished. Instead of assembly proteins, the proteins that are then transported are the ones required for the infection process.
Crucial for the impressive results obtained by Marlovits and his team was the combination of high-resolution imaging methods: cryo electro microscopy with the molecular genetic analysis of mutants that form unusually long needle structures. The team knew that the protein InvJ influenced needle length - but how this influence is exerted was not fully understood. Marlovits' comparison yielded a surprisingly clear picture: the mutants lacked the inner ring structure completely. Since these mutants are nevertheless able to form needle structures, and indeed extremely long ones, it was suspected that the inner ring structure provides a type of stop signal for the needle-building process - a signal that the mutants lacked. Further analyses then showed further clear structural differences between the bases of the wild types and those of the mutants. Marlovits' hypothesis is now that this structural change influences the binding of other proteins that are channelled via the TTSS and thus provides the stop signal for the needle-building process.
Original publication: "Assembly of the inner rod determines needle length in the type III secretion injectisome."; Nature 2006, 441, 637-640.
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.