UCB and Immunomedics announce worldwide development collaboration and license agreement for Epratuzumab
UCB and Immunomedics, Inc. announced a collaboration and license agreement for Immunomedics' lead product, epratuzumab. The agreement grants UCB the exclusive worldwide rights to develop, market and sell epratuzumab for all autoimmune disease indications. Epratuzumab's most advanced program is for the treatment of systemic lupus erythematosus (SLE): it has been granted FDA Fast Track designation and is currently undergoing two phase III clinical trials.
Immunomedics will receive initial cash payments totaling 38 million U.S. dollars over the next 10 business days and could receive potential regulatory milestone payments of up to 145 million U.S. dollars in cash and 20 million U.S. dollars in equity investments, depending on geography approval and approval in different indications over several years. In addition to receiving royalties on sales, Immunomedics could also receive sales bonuses upon reaching certain sales target levels.
Epratuzumab, a humanized monoclonal antibody against the CD22 marker expressed on activated B-cells, was developed and manufactured internally at Immunomedics, and is covered by worldwide patent estate. It is Immunomedics' lead product candidate being evaluated in two international pivotal Phase III ("ALLEVIATE A and B") trials for the treatment of moderate and severe SLE. The FDA granted a Fast Track designation to the clinical development program for epratuzumab for the treatment of patients with SLE, following Immunomedics' completion of a Phase II trial.
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Rutgers researcher uncovers new gene for fear factor - Findings could pave the way to treatment of anxiety disorders

What determines the stability of proteins?
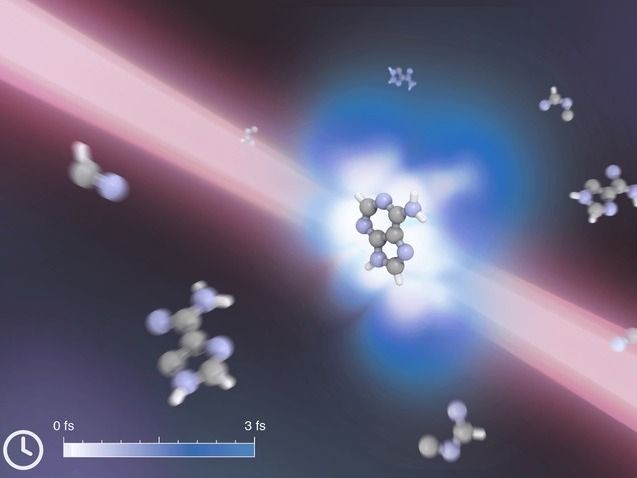
Ultrafast lasers protect adenine - Ultrafast lasers can protect DNA building block from light-induced dissociation
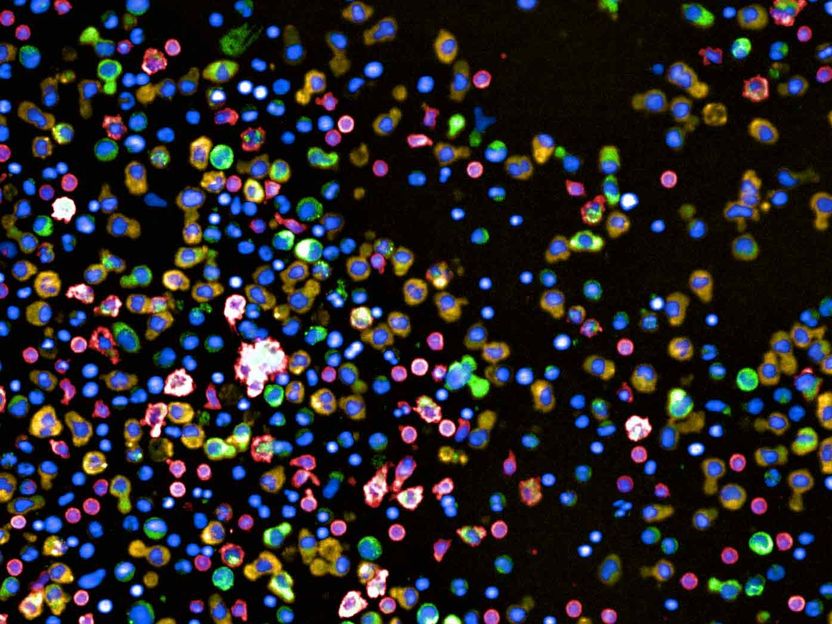
How can we fight blood cancer more effectively? - Several hundred different treatment combinations to be tested simultaneously outside the body

B Medical Systems S.à r.l. - Hosingen, Luxembourg
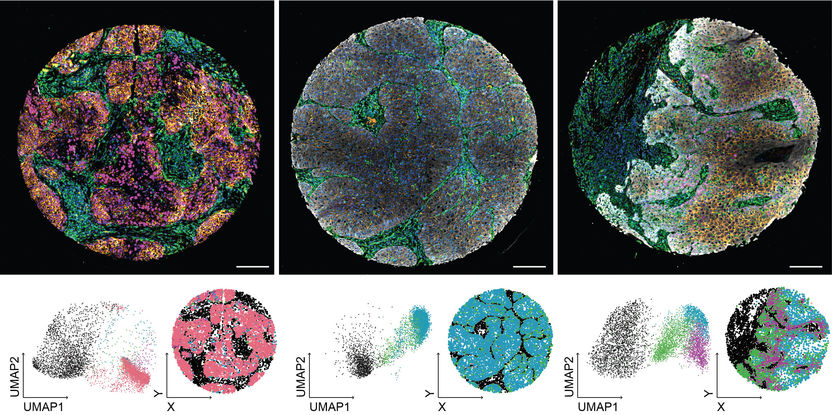
New imaging analysis method revolutionizes the diagnosis of head and neck tumors
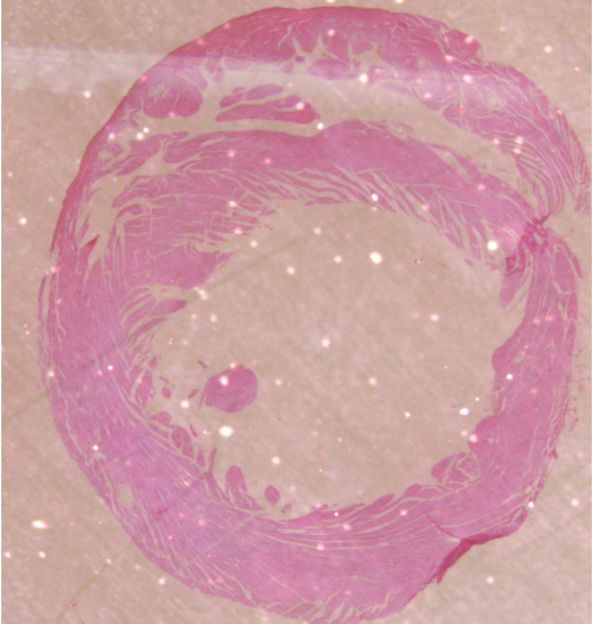
Scientific Paradox: Phosphate Increases The Concentration of Sodium (!) in The Blood
BASF Plant Science takes Amflora case to EU Court - BASF filed action against the EU Commission for failure to act
A double ring ceremony prepares telomerase RNA to wed its protein partner

Using light to produce medication and plastics more efficiently - How the energy efficiency of photochemical reactions can be increased tenfold
AMT's Lead Product Poised to Address Major Liver Disease - European Patent Office Grants Patent for Treatment of Non-alcoholic Steatotic Hepatitis




















































