Novavax Successfully Completes Additional Vaccine Milestone
Validation of cGMP Biological Manufacturing Facility Complete
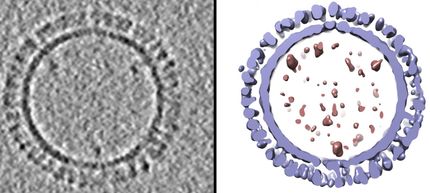
Novavax, Inc. announced that it has successfully completed the renovation and validation for current "Good Manufacturing Practices" (cGMP) compliance of it's industrial facility located in Rockville, Maryland. This validation will allow production of clinical grade materials of its virus-like particle (VLP) vaccine candidates against H5N1 and other pandemic influenza as well as for human seasonal influenza. The facility, located at One Taft Court, Rockville, Maryland, was released for manufacture of biological products in compliance with current U.S. food and Drug Administration (FDA) standards by a leading independent consulting firm.
"To validate a facility compliant with current GMP standards is a difficult and challenging hurdle and can become a rate-limiting step in the development of vaccines. We are delighted to have initiated the cGMP manufacture of VLP influenza vaccines well ahead of schedule. With a validated cGMP production capability now in-house, we can produce the requisite clinical trial materials in an efficient and timely manner to support the human testing planned of our pandemic and seasonal influenza vaccine programs. Clinical manufacturing at our Rockville facility will allow us to now complement the preparation work being conducted at our partner's PacificGMP's facility located in San Diego, California," stated Dr. Gale Smith, Vice President of Vaccine Development at Novavax.
Most read news
Other news from the department manufacturing

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.