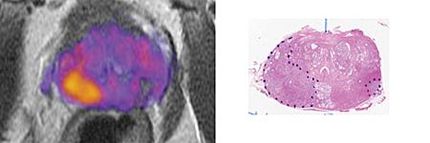
Algeta's Alpharadin has potential as a treatment for bone metastases in prostate cancer patients
Algeta ASA, a therapeutics company dedicated to the development of novel anticancer agents based on alpha particle emitting radionuclides, announced that an analysis of the biomarker data from a Phase II trial of its lead product Alpharadin(TM) (radium-223) continues to demonstrate its potential as a treatment for bone metastases in hormone-refractory prostate cancer (HRPC) patients. Data from this trial (BC1-02) were presented at the 2006 Prostate Cancer Symposium in San Francisco, CA.
Algeta is conducting trial BC1-02 as part of its Phase II clinical program for Alpharadin(TM), a novel radiopharmaceutical based on the alpha particle emitter radium-223, which naturally targets and attacks skeletal metastases. The double-blind placebo-controlled trial involves 64 patients with painful skeletal metastases as a consequence of HRPC and is in its follow-up phase at 11 centers in Norway, Sweden and the UK. The trial was fully enrolled in May 2005.
The trial results presented are based on four-month follow-up data. Alpharadin(TM) treatment met the primary endpoint of the trial. There was a highly statistically significant decrease of bone-alkaline phosphatase (bone-ALP) compared to placebo (ITT: p<0.001). Strong demonstration of Alpharadin's effect on other markers of bone turnover, S-PINP (bone formation) and S-CTX-I and S-ICTP (bone resorption), were also observed.
Interesting PSA (prostate specific antigen) results were demonstrated with a significantly better PSA response in patients given Alpharadin(TM) compared to placebo. Together, these data show that Alpharadin(TM) treatment has a clear effect on the microenvironment of bone metastases indicative of a positive therapeutic effect.
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Drug costs vary by more than 600% in study of 10 high-income countries
Mimicking biological process, hydrogel signals and releases proteins
GE Healthcare to acquire PAA Laboratories GmbH
Precursor Cells Generated From Human Embryonic Stem Cells Show Ability to Repair Vascular Damage in Animals - New, scalable population of hemangioblast cells halves the death rate following heart attack and repairs ischemic limbs and damaged vasculature