CellGenesys Reports Encouraging Follow-Up Survival Data From Second Phase 2 Trialof GVAX Immunotherapy for Prostate Cancer
Cell Genesys, Inc. reported updated results from itssecond multi-center Phase 2 trial of GVAX(R) immunotherapy for prostate cancerin patients with advanced metastatic hormone-refractory prostate cancer (HRPC). Additional follow-up of thepatients who received the dose that is comparable to that being employed in the company's ongoing Phase 3 program indicates that the median survival has not yet been reached and that the estimated median survival will be no less than29.1 months. The company also presented for the first time, an analysis of the survival data for the company's two Phase 2 trials of GVAX immunotherapy for prostate cancer, based on a published,validated nomogram that uses seven prognostic factors to calculate a patient's predicted survival. This analysis demonstrated a favorable comparison of observed to predicted median survival for both Phase 2 trials. These newfindings were reported at the 2006 American Society of Clinical oncology (ASCO) Prostate Cancer Symposium in San Francisco, CA, by Eric J.Small, M.D., professor of medicine and urology, University of California SanFrancisco Comprehensive Cancer Center.
Cell Genesys is currently enrolling two Phase 3 clinical trialsof GVAX immunotherapy for prostate cancer in approximately 1200 patients with metastatic HRPC, comprising one of the largest Phase 3 clinical programs ever conducted in men with advanced prostate cancer. The first trial (VITAL-1) is enrolling chemotherapy naive,asymptomatic patients without cancer-related pain and will compare GVAX cancerimmunotherapy to Taxotere chemotherapy plus prednisone. The second trial (VITAL-2) is enrolling patients who are symptomatic with cancer-related pain and will compare GVAXcancer immunotherapy plus Taxotere chemotherapy to Taxotere plus prednisone. Each Phase 3 trial is expected to enroll 600 patients and is designed to demonstrate a survival benefit compared to Taxotere plus prednisone. Cell Genesys received Special Protocol Assessments (SPA) from the U.S.Food and Drug Administration (FDA) for each of the VITAL-1 and VITAL-2 Phase 3 studies.
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Society for Clinical Data Management (SCDM) - Brüssel, Belgium
Category:KAZAL_domain
Stealth-adapted_virus
Scientists spin artificial silk from whey protein - X-ray study throws light on key process for production
When Tooth Decay Becomes Fatal - Researchers to study role of oral streptococci in serious diseases
Category:Orthodontic_Technicians_Association
Advanced Cell Technology Announces Creation of Human Embryonic Stem Cell Lines Without the Destruction of Embryos - Breakthrough Approach Improves Efficiency to Levels Reported in the Conventional Stem Cell Derivation Techniques
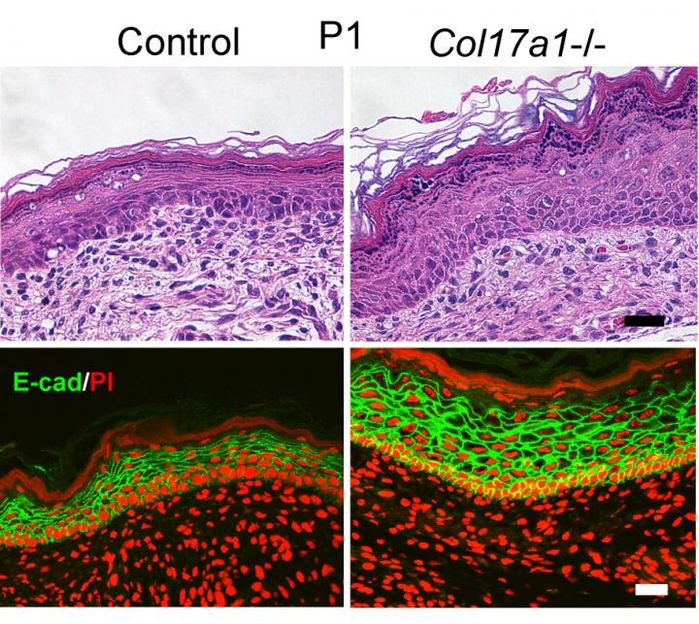
Collagen controlling the thickness and juvenile state of skin
Journal_of_Bone_&_Joint_Surgery
Category:Marine_biologists





















































