Dendreon Announces Strategic Realignment to Focus on PROVENGE BLA and Commercialization
Dendreon Corporation announced the Company has realigned its resources to focus on advancing PROVENGE® (sipuleucel-T), its lead investigational active cellular immunotherapy for the treatment of prostate cancer, toward the market as expeditiously as possible. In addition, Michelle Burris, senior vice president and chief financial officer, will be leaving the Company. Rick Hamm, senior vice president, corporate development and general counsel will now oversee financial functions and David Urdal, Ph.D., senior vice president and chief scientific officer, will oversee manufacturing operations.
The majority of the Company's resources will be deployed to complete the Biologics License Application (BLA) and prepare for the commercialization of PROVENGE. PROVENGE has Fast Track designation and the Company plans to begin submitting the BLA to the U.S. Food and Drug Administration (FDA) on a rolling basis by the middle of this year. The Company will apply for Priority Review upon completion of its BLA submission later this year. The BLA is based on randomized Phase 3 data that showed a statistically significant survival benefit with PROVENGE in the overall intent-to-treat group of men with advanced prostate cancer. In addition, the Company will also focus on completing an ongoing Phase 3 study, known as the IMPACT (IMmunotherapy for Prostate AdenoCarcinoma Treatment) trial, for the potential treatment of patients with metastatic, androgen-independent prostate cancer.
As a result of this realignment, Dendreon reduced its workforce by 15 percent or 34 employees, primarily in early-stage research and development and in general and administrative functions. The Company plans to continue hiring highly specialized and skilled individuals needed to commercialize PROVENGE, particularly at its manufacturing plant in East Hanover, NJ.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Category:Parvoviruses
Next-generation vascular stents
New Business Development Appointments Strengthen ClinPhone's Operations
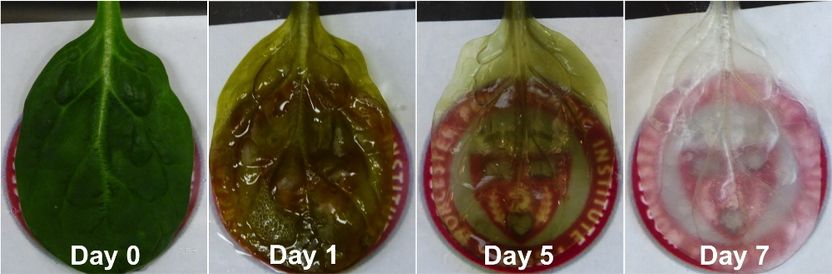
Growing heart tissue on spinach leaves