Rutgers researcher uncovers new gene for fear factor
Findings could pave the way to treatment of anxiety disorders
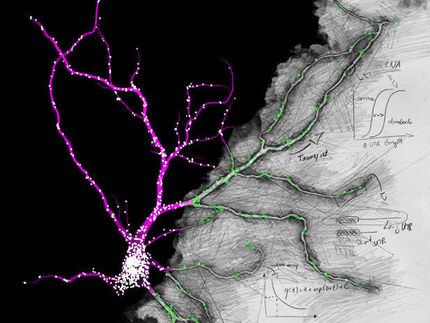
Rutgers geneticist Gleb Shumyatsky has discovered a gene that controls both innate and learned forms of fear. The gene, known as Stathmin or Oncoprotein 18, is highly concentrated in the amygdala, a key region of the brain that deals with fear and anxiety.
"This is a major advance in the field of learning and memory that will allow for a better understanding of post traumatic stress disorder, phobias, borderline personality disorder and other human anxiety diseases," said Shumyatsky, an assistant professor of genetics at Rutgers, The State University of New Jersey. "It will provide important information on how learned and innate fear is experienced and processed, and may point the way to apply new therapies."
In collaboration with Nobel laureate Eric Kandel at Columbia University and Vadim Bolshakov at Harvard Medical School, Shumyatsky had previously identified another gene that controlled learned but not innate fear. The new research being reported by Shumyatski, Kandel et al. is the first major attempt to analyze how both learned and innate fear is controlled at the molecular level.
Shumyatsky, his collaborators and their laboratory colleagues have been able to correlate changes in the expression of Stathmin to changes in short-term or long-term strength of nerve impulses and fear responses. They relied on a combination of mouse genetics, cellular electrophysiology and behavior. The team's collaborative findings are presented in the Nov. 18 issue of the journal Cell Online.
Stathmin knockout mice, or mutants bred to be deficient in this gene, showed an increase in the amount of microtubules. These are the building blocks of the dendrite skeleton and also serve as paths for certain proteins to follow, proteins that govern the strength of the connections between neurons (synapses). In the absence of Stathmin, microtubule dynamics (meaning the speed and flexibility of building these paths) are likely to be decreased and may lead to the weakening of the synaptic connections.
This is consistent with a significant reduction in long-term potentiation or LTP - the lasting, strengthened electrical connections between neurons that are regarded as a molecular model for memory. The reduction was specifically observed in pathways incoming to the amygdala in the knockout mice.
The microtubule increase, and the LTP decrease, may be at the root of the noted failure in the mice to remember the lessons of learned fear, such as avoiding places that gave electric shocks. In addition, the researchers analyzed Stathmin-deficient mice for their anxiety levels by recording their performance in mazes. Mice instinctively avoid open spaces, but the knockout mice showed no fear and consistently explored more open areas than normal mice. Thus, reductions in innate fear behaviors, such as avoiding open spaces as opposed to "safer" areas with less exposure, correlated with the absence of Stathmin.
Shumyatsky explained that the difference between the earlier research paper and the current one is that the first described a gene that is expressed in the learned fear circuitry and controls ONLY learned fear but not innate fear. The new paper describes a gene that controls both learned AND innate fear. This work therefore emphasizes the importance of local gene expression in the neural circuits responsible for specific behaviors. In addition, Shumyatsky said that the gene is a negative regulator of microtubule formation and consequently microtubule dynamics are important for fear expression and fear learning.
"This study provides genetic evidence that amygdala-enriched Stathmin is required for the expression of innate fear and the formation of memory for learned fear," the authors concluded. "Stathmin knockout mice can be used as a model of anxiety states of mental disorders with innate and learned fear components (and) these animal models could be used to develop new anti-anxiety agents," they added.
Most read news
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Ice-free through the winter - Formic acid enables environmentally friendly deicing of runways and roads – and is successful in many other applications
Sigma Life Science Announces Scientific Advisory Board to drive ADME/Tox program - Board to Provide Counsel on New Solutions for Pre-clinical Testing with Sigma’s CompoZr® Zinc Finger Nuclease (ZFN) Technology