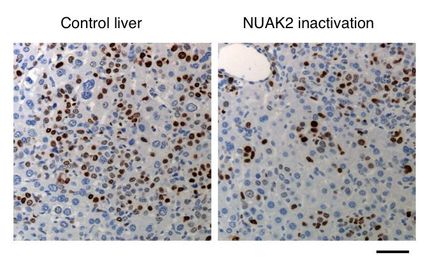
BrachySil(TM) Phase IIb Clinical Trials for Liver Cancer Commence Following Approval by HSA
First patient in multi-centre dose-profiling study receives treatment in Singapore General Hospital after approval by the Health Sciences Authority
pSivida Limited announced that Phase IIb clinical trials have commenced with BrachySil(TM) (32-P BioSilicon(TM)) as a potential new brachytherapy treatment for inoperable primary liver cancer (hepatocellular carcinoma, HCC). The first patient has successfully received treatment at Singapore General Hospital ("SGH") using a new fine-gauge needle, multi-injection device which will enable for the first time, larger and also multiple tumors to be treated. A total of 50 patients will be entered into this multi-centre trial, which will be conducted in Singapore, Malaysia and Vietnam.
The study, which was designed in collaboration with SGH and approved by the Singaporean regulatory authority (Health Sciences Authority) will determine the optimal dose of BrachySil(TM) in treating inoperable HCC. Patients will be evaluated up to 12 months after treatment, and the endpoints are based on evaluations of patient safety and target tumor responses, as well as overall survival.
The study is intended to provide pivotal efficacy and safety data to support future product registration and approval of BrachySil(TM) as an effective treatment for primary liver cancer. These results are expected to build on the findings of a Phase IIa study concluded earlier this year on patients with advanced liver cancer. In this trial, which was also conducted at the SGH, BrachySil(TM) was found to be both safe and well tolerated. It was also found to reduce significantly the size of some tumors treated even on a lower dose as used in the earlier trials.
In addition, the Phase IIb trial will include a clinical evaluation of pSivida's proprietary SIMPL(TM) implantation system. SIMPL(TM) is a fine-gauge needle, multi-injection device, through which BrachySil(TM) is injected as a liquid suspension directly into tumors under local anesthetic. The device has been designed to ensure the effective distribution of the implanted dose from a single entry point and, for the first time, to enable physicians to treat larger tumors. This device is expected to be a further significant advantage of BrachySil(TM) over existing brachytherapies as well as assist in expanding the application of BrachysilTM to other solid tumour cancers, in addition to pancreatic cancer for which a phase IIa trial is currently being planned.
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Case solved: the biosynthesis of strychnine elucidated - New possibilities for the production of previously unknown plant natural products using "metabolic engineering" approaches
Cross species transfer of genes has driven evolution
The Hastings Center - Garrison, USA
CSHL structural biologists reveal molecular architecture of key NMDA receptor subunit - Structure of GluN2D subunit when docked with certain neurotransmitters helps explain the receptor's slow deactivation
Microbial murder mystery solved
Biomass for biofuels, biochemicals will more than triple to 3.7 billion tons in 2030